What Functional Groups Are In Aspirin
News Leon
Mar 25, 2025 · 5 min read
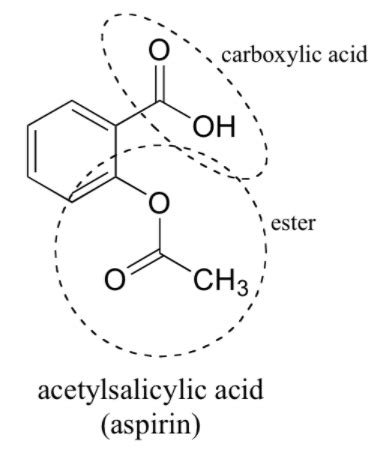
Table of Contents
What Functional Groups Are in Aspirin? A Deep Dive into the Chemistry of a Common Drug
Aspirin, a household name synonymous with pain relief, fever reduction, and inflammation control, is more than just a simple over-the-counter medication. Understanding its chemical structure, specifically the functional groups present, is key to grasping its pharmacological properties and mode of action. This comprehensive article delves deep into the fascinating chemistry of aspirin, exploring its constituent functional groups and their contributions to its overall activity.
Aspirin's Chemical Structure: A Foundation for Understanding
Aspirin, chemically known as acetylsalicylic acid, boasts a relatively simple yet impactful molecular structure. This structure is the cornerstone of its pharmacological activity, and its functional groups dictate how it interacts with the body. The chemical formula for aspirin is C₉H₈O₄.
Its structure can be represented in various ways:
O
||
CH3-C-O-C-C6H4-COOH
|
H
or a more simplified structural formula:
CH3COOC6H4COOH
This seemingly simple structure contains several crucial functional groups, each playing a vital role in its properties and physiological effects.
Deconstructing Aspirin: Identifying the Key Functional Groups
Three major functional groups are crucial to aspirin's characteristics and mechanism of action:
1. Ester Functional Group (-COO-)
The most prominent feature of aspirin's structure is the ester functional group. This group is formed by the reaction of a carboxylic acid (acetic acid) and an alcohol (salicylic acid). The ester linkage connects the acetyl group (CH₃CO-) to the salicylic acid moiety. The presence of this ester is critical because it's the site of hydrolysis in the body, converting aspirin back into salicylic acid, its active form. This hydrolysis is catalyzed by enzymes, primarily esterases, present in the body. The speed of this hydrolysis directly impacts the onset and duration of aspirin's effects. The ester linkage is essential for aspirin's stability; it’s less reactive than the free carboxylic acid group of salicylic acid, making it relatively stable on the shelf.
Importance of the Ester in Aspirin's Pharmacology:
- Hydrolysis to Salicylic Acid: The ester group is cleaved in the body, releasing salicylic acid, the pharmacologically active component of aspirin.
- Enhanced Absorption and Bioavailability: The ester modification increases the lipophilicity (fat-loving nature) of the molecule, facilitating better absorption from the gastrointestinal tract compared to salicylic acid.
- Reduced Gastric Irritation: While salicylic acid is known to cause gastric irritation, the ester modification mitigates this effect to some extent, contributing to improved tolerability.
2. Carboxylic Acid Functional Group (-COOH)
The carboxylic acid functional group is another cornerstone of aspirin's structure. This group is present within the salicylic acid component of the molecule. The carboxylic acid group is responsible for several key properties:
Importance of the Carboxylic Acid in Aspirin's Pharmacology:
- Acidity: This group contributes to aspirin's acidic nature, affecting its solubility and absorption in the body. The acidity allows it to be partially ionized in the stomach’s acidic environment, enhancing absorption.
- Interaction with Biological Targets: The carboxylic acid group plays a role in how aspirin interacts with its targets within the body, specifically cyclooxygenase (COX) enzymes.
- Salt Formation: The carboxylic acid group enables the formation of various aspirin salts, which can alter the drug's properties, such as solubility and absorption rate.
3. Aromatic Ring (Benzene Ring)
The aromatic ring (specifically, a benzene ring) forms the backbone of the salicylic acid component. The presence of this aromatic system contributes significantly to the overall stability and reactivity of the molecule. Its electron delocalization influences the acidity of the carboxylic acid group and affects how aspirin interacts with its biological targets.
Importance of the Aromatic Ring in Aspirin's Pharmacology:
- Stability: The aromatic ring imparts stability to the molecule, contributing to its shelf-life and resistance to degradation.
- Planarity: The planar structure of the aromatic ring influences the way aspirin interacts with its biological targets through spatial interactions and steric effects.
- Electronic Effects: The electron-rich nature of the aromatic ring affects the reactivity of other functional groups within the molecule.
Aspirin's Mechanism of Action: The Role of Functional Groups
Aspirin's primary mechanism of action involves the inhibition of cyclooxygenase (COX) enzymes. COX enzymes are involved in the biosynthesis of prostaglandins, thromboxanes, and prostacyclins – potent mediators of inflammation, pain, and fever.
Aspirin, after hydrolysis to salicylic acid, acts as a non-selective inhibitor of COX-1 and COX-2. This inhibition reduces the production of these inflammatory mediators, leading to its analgesic (pain-relieving), antipyretic (fever-reducing), and anti-inflammatory effects. The carboxylic acid group of salicylic acid plays a crucial role in interacting with the active site of the COX enzymes, facilitating this inhibition. The benzene ring also influences the binding affinity and specificity.
Functional Group Modifications and Aspirin Analogues
Modifying the functional groups within aspirin's structure can lead to the development of new drugs with altered pharmacological properties. For example, changing the acetyl group or the substituents on the benzene ring could potentially lead to variations in COX selectivity, effectiveness, and side effect profiles. Researchers continue to explore such modifications to improve existing pain-relieving medications or develop new therapeutic approaches.
Safety and Side Effects: A Functional Group Perspective
The functional groups in aspirin also contribute to potential side effects. The carboxylic acid group’s acidity can lead to gastric irritation, a common side effect. The interaction of aspirin with COX-1 (which is present in the gastrointestinal tract) can increase the risk of bleeding. Modifications to the molecule aimed at reducing COX-1 inhibition are explored to minimize this risk.
Conclusion: A Chemical Perspective on a Common Drug
Aspirin's simple yet powerful structure contains several crucial functional groups. The ester, carboxylic acid, and aromatic ring each play critical roles in its absorption, metabolism, and interaction with biological targets. A deep understanding of these functional groups, their interactions, and their influence on aspirin's activity is vital for appreciating its pharmacological significance and for the development of future analgesic and anti-inflammatory medications. Further research continues to explore the nuances of aspirin’s chemistry and refine its use in treating a variety of conditions. The exploration of functional group modifications holds the key to creating even more effective and safer pain relief options in the future.
Latest Posts
Latest Posts
-
Give The Major Product For The Following Reaction
Mar 28, 2025
-
Is India In The Northern Or Southern Hemisphere
Mar 28, 2025
-
Predict The Major Product S Of The Following Reaction
Mar 28, 2025
-
Find The Value Of X In The Figure Below
Mar 28, 2025
-
9 Is What Percent Of 16
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Functional Groups Are In Aspirin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
