Predict The Major Product S Of The Following Reaction
News Leon
Mar 28, 2025 · 6 min read
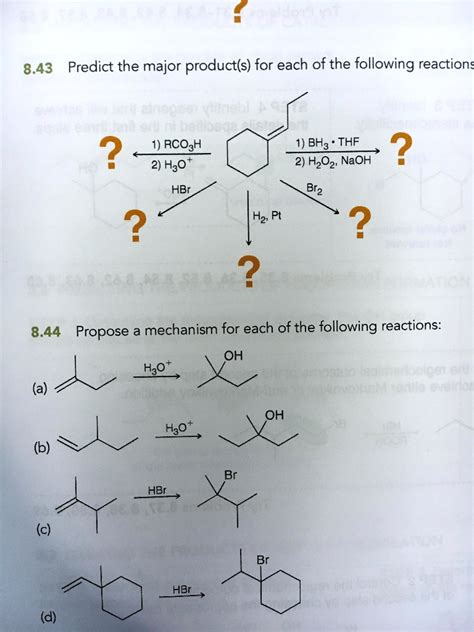
Table of Contents
Predicting the Major Products of Organic Reactions: A Comprehensive Guide
Predicting the major product of an organic reaction is a crucial skill for any organic chemist. It requires a deep understanding of reaction mechanisms, functional group reactivity, and the influence of various reaction conditions. This comprehensive guide delves into the key principles and strategies involved in accurately predicting the major products of various organic reactions. We'll explore different reaction types, analyze the factors that influence product formation, and provide practical examples to solidify your understanding.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before we can predict the major product, we must understand the mechanism of the reaction. The mechanism details the step-by-step process of bond breaking and bond formation. Knowing the mechanism allows us to identify the intermediate species, which are crucial in determining the final products. Different mechanisms lead to different products, even with the same starting materials.
Key mechanistic concepts for prediction:
- Nucleophilic Attack: Nucleophiles, electron-rich species, attack electron-deficient centers (electrophilic centers). Understanding the nucleophile's strength and the electrophile's susceptibility to attack is vital.
- Electrophilic Attack: Electrophiles, electron-deficient species, attack electron-rich centers (nucleophilic centers). The electrophile's reactivity and the nucleophile's availability significantly influence the outcome.
- Carbocation Stability: Carbocations (positively charged carbon atoms) are common intermediates in many reactions. Their stability (tertiary > secondary > primary > methyl) dictates the direction of the reaction. More stable carbocations are formed preferentially.
- Steric Hindrance: Bulky groups can hinder the approach of reactants, affecting reaction rates and regioselectivity (where the reaction occurs on the molecule).
- Resonance Stabilization: Resonance structures can delocalize charge and stabilize intermediates, influencing product formation.
Common Reaction Types and Product Prediction
Let's examine some common reaction types and the factors that govern product prediction:
1. SN1 and SN2 Reactions: Nucleophilic Substitution
These reactions involve the substitution of one group by a nucleophile. The difference lies in the mechanism:
-
SN1 (Unimolecular Nucleophilic Substitution): Proceeds through a carbocation intermediate. The rate depends only on the concentration of the substrate. Favored by tertiary alkyl halides and polar protic solvents. Often leads to racemization (loss of chirality) due to the planar nature of the carbocation. Predicting the major product: Focus on the stability of the carbocation intermediate. The most stable carbocation will lead to the major product.
-
SN2 (Bimolecular Nucleophilic Substitution): Proceeds through a concerted mechanism (one step). The rate depends on the concentration of both the substrate and the nucleophile. Favored by primary alkyl halides and polar aprotic solvents. Leads to inversion of configuration (stereochemistry). Predicting the major product: Consider steric hindrance. Less hindered substrates react faster. Stronger nucleophiles favor SN2 reactions.
Example: The reaction of 2-bromobutane with sodium hydroxide (NaOH) in ethanol (a polar protic solvent) would likely proceed via SN1 to form a mixture of 2-butanol (racemic) and but-2-ene (elimination product). However, in a polar aprotic solvent like DMSO, SN2 would be favored yielding predominantly (S)-2-butanol (inverted stereochemistry).
2. E1 and E2 Reactions: Elimination Reactions
These reactions involve the removal of a leaving group and a proton from adjacent carbons, resulting in the formation of a double bond (alkene).
-
E1 (Unimolecular Elimination): Proceeds through a carbocation intermediate. The rate depends only on the concentration of the substrate. Favored by tertiary alkyl halides and polar protic solvents. Often leads to a mixture of alkenes (Zaitsev's rule: the more substituted alkene is usually the major product). Predicting the major product: Focus on the stability of the alkene formed (more substituted alkenes are more stable).
-
E2 (Bimolecular Elimination): Proceeds through a concerted mechanism. The rate depends on the concentration of both the substrate and the base. Favored by strong bases and can occur with primary, secondary, and tertiary alkyl halides. Predicting the major product: Consider the orientation of the base and the substrate. Anti-periplanar arrangement is preferred. Zaitsev’s rule often applies.
Example: Dehydration of 2-methyl-2-propanol using concentrated sulfuric acid (E1 mechanism) primarily yields 2-methylpropene (the more substituted alkene, following Zaitsev's rule).
3. Addition Reactions: Addition to Alkenes and Alkynes
These reactions involve the addition of atoms or groups to a carbon-carbon double or triple bond. The regioselectivity (where the addition occurs) and stereoselectivity (which stereoisomer is formed) are crucial for product prediction.
- Markovnikov's Rule: In the addition of HX (hydrogen halide) to an alkene, the hydrogen atom adds to the carbon atom that already has more hydrogen atoms. This is due to the formation of a more stable carbocation intermediate.
- Anti-Markovnikov Addition: Certain radical additions (e.g., using HBr with peroxides) follow anti-Markovnikov's rule. The hydrogen atom adds to the carbon atom with fewer hydrogen atoms.
Example: The addition of HBr to propene will yield 2-bromopropane (Markovnikov addition). However, adding HBr in the presence of peroxides would yield 1-bromopropane (anti-Markovnikov addition).
4. Grignard Reactions: Nucleophilic Addition to Carbonyl Compounds
Grignard reagents (RMgX) are powerful nucleophiles that add to carbonyl compounds (aldehydes, ketones, esters, etc.). The product depends on the carbonyl compound used.
Example: The reaction of a Grignard reagent (e.g., CH3MgBr) with formaldehyde (HCHO) will yield a primary alcohol. Reaction with an aldehyde yields a secondary alcohol, and reaction with a ketone yields a tertiary alcohol.
5. Oxidation and Reduction Reactions
These reactions involve the gain or loss of electrons. Predicting the product requires understanding the oxidizing or reducing agent's strength and the substrate's reactivity.
Example: Oxidation of a primary alcohol with strong oxidizing agents like potassium permanganate (KMnO4) usually yields a carboxylic acid. Oxidation of a secondary alcohol typically yields a ketone.
Factors Influencing Product Distribution:
Besides the reaction mechanism, several other factors influence the major product formed:
- Temperature: Higher temperatures often favor elimination reactions over substitution reactions.
- Solvent: Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 and E2 reactions.
- Concentration of Reactants: High concentrations of nucleophiles or bases favor SN2 and E2 reactions, respectively.
- Steric Effects: Bulky groups can hinder reactions and affect product selectivity.
- Acid/Base Catalysis: Acidic or basic conditions can significantly alter the reaction pathway and product distribution.
Advanced Techniques for Product Prediction:
For more complex reactions, advanced techniques are often necessary:
- Computational Chemistry: Software can predict reaction pathways and energetics, aiding in product prediction.
- Spectroscopic Analysis (NMR, IR, Mass Spectrometry): These techniques are crucial for identifying and characterizing the products obtained experimentally.
Conclusion:
Predicting the major product of an organic reaction is a multifaceted process that requires a thorough understanding of reaction mechanisms, functional group reactivity, and the influence of various reaction conditions. This guide has provided a foundation for making accurate predictions. Mastering this skill is essential for success in organic chemistry, enabling the design and synthesis of specific target molecules. Remember that practice is key – work through numerous examples, and don't hesitate to consult reference materials and utilize advanced techniques when dealing with more complex reactions. Consistent practice and a solid grasp of the underlying principles will significantly enhance your ability to predict the major products of organic reactions with confidence.
Latest Posts
Latest Posts
-
True Or False Evaporation Is A Physical Change
Mar 31, 2025
-
Do Gram Positive Bacteria Have Porins
Mar 31, 2025
-
Which Of The Following Compounds Is Most Soluble In Water
Mar 31, 2025
-
Part Of The Brain That Controls Breathing And Heartbeat
Mar 31, 2025
-
This Pair Of Structures Anchors The Spindle
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Predict The Major Product S Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
