What Does Positive Delta H Mean
News Leon
Mar 24, 2025 · 5 min read
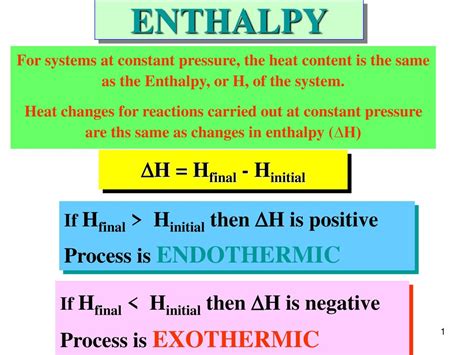
Table of Contents
What Does a Positive Delta H Mean? Understanding Enthalpy Change
Understanding enthalpy and its changes (ΔH) is crucial in chemistry and many other scientific fields. This comprehensive guide delves into the meaning of a positive ΔH, exploring its implications, applications, and how it relates to other thermodynamic concepts.
What is Enthalpy (H)?
Before we dive into the significance of a positive ΔH, let's first clarify what enthalpy (H) represents. Enthalpy is a thermodynamic property of a system, essentially the total heat content of the system at constant pressure. It's a state function, meaning its value depends only on the system's current state (temperature, pressure, etc.), not on the path taken to reach that state. We can't directly measure enthalpy, but we can measure changes in enthalpy (ΔH).
Understanding Delta H (ΔH): The Enthalpy Change
Delta H (ΔH), or enthalpy change, represents the difference in enthalpy between the final and initial states of a system undergoing a process. It quantifies the heat absorbed or released during a process at constant pressure. This is frequently the case in many chemical reactions and physical changes carried out in open containers.
The equation for calculating ΔH is:
ΔH = H<sub>final</sub> - H<sub>initial</sub>
Where:
- H<sub>final</sub> is the enthalpy of the system in its final state.
- H<sub>initial</sub> is the enthalpy of the system in its initial state.
The Significance of a Positive Delta H
A positive ΔH signifies that the system has absorbed heat from its surroundings during the process. In other words, the reaction or process is endothermic. The surroundings lose heat, resulting in a decrease in the temperature of the surroundings. Think of it like this: the system is gaining energy, drawing it from its environment.
Examples of Processes with Positive ΔH:
- Melting ice: To melt ice, you need to supply heat. The ice absorbs heat from its surroundings (e.g., the air) to change its phase from solid to liquid. This is an endothermic process with a positive ΔH.
- Boiling water: Similarly, boiling water requires the input of heat. The water absorbs heat to transition from liquid to gas, demonstrating an endothermic process with a positive ΔH.
- Photosynthesis: Plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This is a highly endothermic process vital for life.
- Many chemical reactions: Many chemical reactions, such as the decomposition of certain compounds, require heat input to proceed, resulting in a positive ΔH.
Differentiating Between Endothermic (Positive ΔH) and Exothermic (Negative ΔH) Processes
It's crucial to contrast endothermic processes (positive ΔH) with exothermic processes (negative ΔH). In exothermic processes, the system releases heat to its surroundings, causing an increase in the surroundings' temperature. Examples include combustion reactions (e.g., burning wood) and many neutralization reactions. The key difference lies in the direction of heat flow:
- Endothermic (ΔH > 0): Heat flows into the system from the surroundings.
- Exothermic (ΔH < 0): Heat flows from the system to the surroundings.
Factors Affecting Delta H
Several factors influence the magnitude of ΔH for a given process:
- The nature of reactants and products: The specific chemical composition of the substances involved significantly affects the enthalpy change. Different bonds have different energies, and breaking and forming bonds contributes to the overall ΔH.
- Temperature: ΔH is often temperature-dependent. While many reactions have ΔH values that are relatively constant over a moderate temperature range, the change in enthalpy can vary with significant temperature shifts.
- Pressure: For processes involving gases, pressure can also influence ΔH.
- State of matter: The physical state of reactants and products (solid, liquid, gas) affects ΔH. Phase transitions (melting, boiling, etc.) have associated enthalpy changes.
Applications of Understanding Positive Delta H
The concept of a positive ΔH, and its understanding as an indicator of endothermic processes, has numerous applications across diverse fields:
1. Chemistry:
- Reaction spontaneity: While ΔH provides information about the heat flow, it alone doesn't determine if a reaction will spontaneously occur. Other thermodynamic factors, like entropy (ΔS) and Gibbs Free Energy (ΔG), must be considered to predict spontaneity. A positive ΔH doesn't automatically mean a reaction is non-spontaneous; the overall Gibbs Free Energy change determines spontaneity.
- Reaction kinetics: Understanding the heat required (or released) helps in designing and controlling chemical reaction conditions.
- Thermochemical calculations: ΔH values are essential for performing various thermochemical calculations, such as determining the enthalpy changes of complex reactions using Hess's Law.
2. Physics:
- Phase transitions: Understanding the enthalpy changes during phase transitions (melting, freezing, vaporization, condensation, sublimation, deposition) is vital in many physical phenomena and engineering applications.
- Heat transfer calculations: Positive ΔH values are critical in calculating the amount of heat needed for endothermic phase changes or processes.
3. Biology:
- Metabolic processes: Many biological processes, like photosynthesis, are endothermic. Understanding the enthalpy changes involved helps in studying metabolic pathways and energy balance in organisms.
- Enzyme kinetics: Enzyme-catalyzed reactions often involve enthalpy changes, which influence the reaction rates.
4. Engineering:
- Chemical engineering: Designing and optimizing chemical reactors requires understanding the heat transfer associated with endothermic and exothermic reactions.
- Material science: The enthalpy changes during material processing (e.g., melting, alloying) influence material properties.
Measuring Delta H: Calorimetry
Experimental determination of ΔH often involves calorimetry. A calorimeter measures the heat flow during a process. The type of calorimeter used depends on the nature of the process and the desired precision. Simple calorimeters measure heat changes at constant pressure, while more sophisticated instruments can control other parameters.
Conclusion
A positive ΔH signifies an endothermic process, indicating that the system absorbs heat from its surroundings. This simple yet powerful concept underpins many natural phenomena and is crucial across various scientific and engineering disciplines. Understanding enthalpy changes and their implications is fundamental for predicting reaction behavior, designing experiments, and solving practical problems in diverse fields. By mastering the concept of positive ΔH, you unlock a deeper understanding of the energetic transformations that shape our world.
Latest Posts
Latest Posts
-
What Does The Slope Of A Distance Time Graph Represent
Mar 26, 2025
-
What Is The Order Of Rotational Symmetry Of A Rhombus
Mar 26, 2025
-
A Wire With A Resistance Of 6 0 Ohm Is Drawn
Mar 26, 2025
-
Which Unit Has Nothing To Do With Electricity
Mar 26, 2025
-
A Man Standing On The Roof Of A Building
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Does Positive Delta H Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
