What Are Three Parts Of An Atp Molecule
News Leon
Mar 14, 2025 · 6 min read
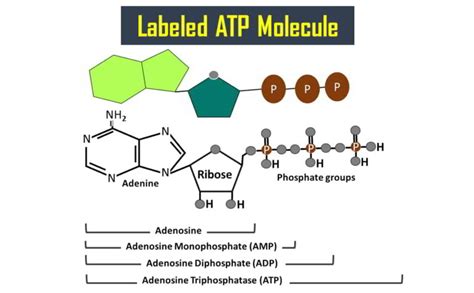
Table of Contents
What Are the Three Parts of an ATP Molecule? Unlocking the Energy Currency of Life
Adenosine triphosphate (ATP) is the fundamental energy currency of all living cells. Understanding its structure is crucial to comprehending how life functions at a molecular level. This comprehensive guide delves into the three crucial components of an ATP molecule, explaining their roles and highlighting the significance of ATP in various biological processes. We'll explore the molecule's structure in detail, its energy-carrying capacity, and its vital roles in cellular functions.
The Tripartite Structure of ATP: Adenine, Ribose, and Triphosphate
The ATP molecule boasts a relatively simple yet remarkably effective structure consisting of three key parts:
- Adenine: A nitrogenous base, a crucial component of DNA and RNA. Its aromatic ring structure contributes to ATP's stability and interactions with enzymes.
- Ribose: A five-carbon sugar molecule (a pentose). Its ring structure forms the backbone of the ATP molecule and provides a crucial point of attachment for both the adenine and the phosphate groups. The specific arrangement of the ribose—a β-D-ribose—is important for the molecule's function.
- Triphosphate: A chain of three phosphate groups (hence, triphosphate). This is the powerhouse of the ATP molecule. The high-energy bonds between these phosphate groups are responsible for ATP's ability to store and release energy.
Let's delve deeper into each component:
1. Adenine: The Nitrogenous Base Foundation
Adenine (A) is a purine base, meaning it's a double-ring structure composed of a six-membered ring fused to a five-membered ring. This specific arrangement of nitrogen and carbon atoms provides unique chemical properties that allow it to participate in hydrogen bonding with other bases, like thymine (T) in DNA or uracil (U) in RNA. In ATP, adenine's role is primarily structural, providing a stable platform for the ribose and phosphate groups. Its planar structure contributes to the overall stability of the ATP molecule and facilitates its interactions with enzymes involved in ATP metabolism. The precise placement and orientation of adenine are crucial for recognition by enzymes that utilize ATP.
2. Ribose: The Sugar Backbone
Ribose is a five-carbon sugar molecule, specifically a β-D-ribose, meaning it's a five-membered ring with the hydroxyl group (-OH) on the carbon atom number 1 positioned above the plane of the ring. This specific configuration is critical. The carbon atoms in ribose are numbered 1' to 5', with the prime notation distinguishing them from the atoms in the adenine base. Ribose serves as the connecting link between adenine and the triphosphate group. It forms a nucleoside (adenosine) when bonded to adenine. The hydroxyl groups on the ribose are important for interactions with water and enzymes, influencing ATP's solubility and reactivity. The precise geometry of the ribose ring ensures the correct spatial arrangement of the adenine and phosphate groups, optimizing the molecule for its energy-transfer function.
3. Triphosphate: The Energy-Rich Tail
The triphosphate group is where the real action happens. It's a chain of three phosphate groups linked together by high-energy phosphoanhydride bonds. These bonds are referred to as "high-energy" because a significant amount of energy is released when they are broken. This energy release fuels a multitude of cellular processes. The phosphate groups are denoted as α, β, and γ, starting from the phosphate group closest to the ribose. The bonds between these phosphate groups are not particularly strong in terms of simple covalent bond strength, but the significant release of free energy upon hydrolysis is a consequence of several factors, including:
- Electrostatic repulsion: The negatively charged phosphate groups repel each other strongly. Breaking the bond between them relieves this repulsion, releasing energy.
- Resonance stabilization: The products of hydrolysis (ADP and inorganic phosphate) are more stable than ATP due to increased resonance stabilization.
- Hydration: The products of hydrolysis interact more favorably with water molecules, further contributing to the energy released.
ATP Hydrolysis: The Energy Release Mechanism
The breakdown of ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi) is called ATP hydrolysis. This process is the primary mechanism by which ATP releases its stored energy:
ATP + H₂O → ADP + Pi + Energy
The energy released during this reaction is not heat; instead, it's used directly to drive various endergonic (energy-requiring) reactions in the cell. This coupling of exergonic (energy-releasing) ATP hydrolysis to endergonic reactions is fundamental to cellular metabolism. Enzymes play a crucial role in facilitating ATP hydrolysis and ensuring the energy is directed to the appropriate cellular process.
The Importance of ATP in Cellular Processes
ATP's role extends far beyond a simple energy storage molecule. It's involved in a vast array of vital cellular functions, including:
- Muscle contraction: ATP provides the energy required for the sliding filament mechanism in muscle cells.
- Active transport: ATP fuels the movement of molecules across cell membranes against their concentration gradients (e.g., the sodium-potassium pump).
- Protein synthesis: ATP is required for the formation of peptide bonds during protein synthesis.
- Nerve impulse transmission: The transmission of nerve impulses relies on ATP-dependent processes.
- DNA replication and repair: ATP is crucial for the processes of DNA replication and repair.
- Cellular signaling: ATP serves as a signaling molecule in certain cellular processes.
- Biosynthesis: ATP provides the energy for the synthesis of various biomolecules.
The list goes on—ATP is arguably the most important molecule in cellular biochemistry.
ATP Synthesis: Regenerating the Energy Currency
The cell is constantly using ATP, so it needs a continuous supply. ATP synthesis primarily occurs through cellular respiration (in aerobic organisms) and fermentation (in anaerobic organisms). In cellular respiration, ATP is generated through oxidative phosphorylation, a process occurring in the mitochondria. Here, the energy released from the breakdown of glucose and other fuel molecules is used to establish a proton gradient across the mitochondrial inner membrane. This gradient drives ATP synthesis through ATP synthase, a remarkable molecular machine.
ATP and Disease: The Consequences of Dysfunction
Disruptions in ATP metabolism can have severe consequences, leading to various diseases and disorders. These can range from rare genetic diseases affecting ATP synthesis to broader conditions associated with mitochondrial dysfunction. Conditions such as mitochondrial myopathies, which affect muscle function, and neurodegenerative diseases, where neuronal cells are impacted, are often linked to impaired ATP production.
Conclusion: ATP – The Master Molecule of Life
The three parts of an ATP molecule—adenine, ribose, and triphosphate—work together in a beautifully orchestrated manner to serve as the fundamental energy currency of life. Understanding its structure and function is crucial for comprehending the intricate processes underpinning all living organisms. From muscle contraction to DNA replication, ATP fuels the remarkable complexity of life itself. Future research into ATP metabolism and related pathways promises to unlock further insights into health and disease, paving the way for novel therapeutic strategies targeting cellular energy production. The seemingly simple structure of ATP belies its profound importance in the grand tapestry of life.
Latest Posts
Latest Posts
-
Integral Of 1 X 2 3
Mar 14, 2025
-
How Many Cubic Centimeters In 1 Cubic Meter
Mar 14, 2025
-
How Many Valence Electrons Does Kr Have
Mar 14, 2025
-
A Flywheel With A Diameter Of 1 20m Is Rotating
Mar 14, 2025
-
Is Al Oh 3 Soluble In Water
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Are Three Parts Of An Atp Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
