What Are Electrically Charged Atoms Called
News Leon
Mar 15, 2025 · 5 min read
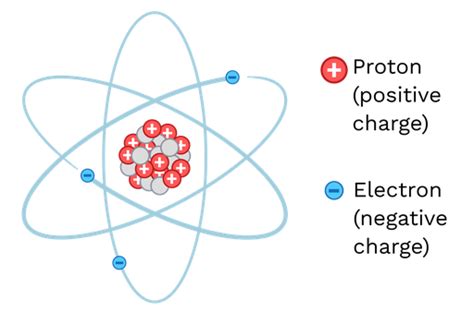
Table of Contents
What Are Electrically Charged Atoms Called? An In-Depth Look at Ions
Electrically charged atoms are called ions. This seemingly simple definition belies a world of fascinating chemistry, physics, and biological processes. Understanding ions is crucial to comprehending a vast range of phenomena, from the behavior of electrolytes in your body to the workings of advanced technologies like batteries and fuel cells. This article will delve deep into the concept of ions, exploring their formation, properties, types, and significant roles in various fields.
The Basics: Atoms, Electrons, and the Birth of Ions
Before diving into the intricacies of ions, let's refresh our understanding of atoms. Atoms are the fundamental building blocks of matter, composed of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by a cloud of orbiting electrons (negatively charged). In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
Ions, however, deviate from this neutrality. They are formed when an atom either gains or loses one or more electrons. This process, known as ionization, fundamentally alters the atom's charge balance.
-
Cation: When an atom loses one or more electrons, it acquires a net positive charge and becomes a cation. Metals tend to form cations because they readily lose electrons to achieve a more stable electron configuration. For example, a sodium atom (Na) loses one electron to become a sodium ion (Na⁺).
-
Anion: Conversely, when an atom gains one or more electrons, it acquires a net negative charge and becomes an anion. Nonmetals often form anions because they readily gain electrons to achieve a more stable electron configuration. For example, a chlorine atom (Cl) gains one electron to become a chloride ion (Cl⁻).
The Driving Force Behind Ion Formation: Achieving Stability
The primary driving force behind ion formation is the pursuit of electronic stability. Atoms strive to achieve a full outer electron shell, a configuration that represents maximum stability. This principle is known as the octet rule, although it's not universally applicable. By gaining or losing electrons, atoms can achieve this stable electron configuration, mimicking the electron arrangement of noble gases, which are exceptionally unreactive.
Consider the example of sodium chloride (NaCl), common table salt. Sodium (Na) has one electron in its outermost shell, while chlorine (Cl) has seven. Sodium readily loses its outermost electron to become Na⁺, achieving a stable electron configuration. Chlorine readily gains this electron to become Cl⁻, also achieving a stable configuration. The electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion forms the ionic bond that holds the salt crystal together.
Types of Ions: A Diverse World
The world of ions is far from uniform. Ions are categorized based on several factors:
Based on Charge:
-
Monoatomic ions: These are simple ions formed from a single atom, such as Na⁺, Cl⁻, Mg²⁺ (magnesium ion), and O²⁻ (oxide ion).
-
Polyatomic ions: These are more complex ions composed of two or more atoms covalently bonded together, carrying a net charge. Examples include:
- Sulfate ion (SO₄²⁻): Found in many minerals and used in various industrial processes.
- Nitrate ion (NO₃⁻): A crucial component in fertilizers and a common pollutant.
- Phosphate ion (PO₄³⁻): Essential for biological processes, including energy transfer and DNA structure.
- Ammonium ion (NH₄⁺): A crucial nitrogen source for plants and found in many cleaning products.
Based on Formation Method:
Ions can be formed through various methods, including:
-
Electron transfer: The most common method, involving the direct transfer of electrons between atoms.
-
Ionization by radiation: High-energy radiation, such as X-rays or gamma rays, can knock electrons out of atoms, creating ions. This process is utilized in various analytical techniques.
-
Chemical reactions: Many chemical reactions involve the formation of ions as reactants or products.
The Significance of Ions: A Wide-Ranging Impact
Ions play crucial roles in a vast array of natural and technological processes:
In Biology:
-
Electrolytes: Ions like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) are crucial electrolytes in biological systems. They regulate fluid balance, nerve impulses, muscle contractions, and many other essential functions. Imbalances in electrolyte levels can have severe health consequences.
-
Enzyme Function: Many enzymes, which catalyze biological reactions, require specific ions for their activity.
-
Protein Structure: Ions can influence the three-dimensional structure of proteins, affecting their function.
In Chemistry:
-
Ionic Compounds: Ions form the basis of ionic compounds, which exhibit characteristic properties like high melting points and solubility in water.
-
Electrochemistry: Electrochemical processes, such as those in batteries and fuel cells, rely on the movement of ions.
-
Chemical Reactions: Many chemical reactions involve the exchange of ions, impacting their rate and equilibrium.
In Technology:
-
Batteries: Batteries utilize the flow of ions between electrodes to generate electricity.
-
Fuel Cells: Fuel cells use electrochemical reactions involving ions to produce electricity efficiently.
-
Semiconductors: The electrical conductivity of semiconductors is influenced by the presence and movement of ions.
-
Sensors: Ion-selective electrodes are widely used as sensors to measure the concentration of specific ions in solutions.
Detecting and Measuring Ions: Analytical Techniques
Several analytical techniques are employed to detect and measure the presence and concentration of ions:
-
Spectroscopy: Techniques like atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS) can identify and quantify ions based on their interaction with light or electromagnetic radiation.
-
Chromatography: Chromatographic methods, such as ion chromatography, separate ions based on their properties and allow for their detection and quantification.
-
Electrochemical methods: Techniques like potentiometry (measuring voltage) and voltammetry (measuring current) can measure ion concentrations based on their electrical properties.
Conclusion: The Ubiquitous Role of Ions
In conclusion, electrically charged atoms, known as ions, are fundamental entities with far-reaching consequences. Their formation, driven by the pursuit of electronic stability, leads to a diverse range of ionic species with unique properties and crucial roles in biology, chemistry, and technology. From the delicate balance of electrolytes in our bodies to the powerful electrochemical reactions in batteries, ions are integral components of the world around us. Continued research and innovation in the field of ion chemistry continue to uncover new applications and deepen our understanding of their fundamental importance. The seemingly simple concept of an electrically charged atom opens a vast and intricate world of scientific exploration.
Latest Posts
Latest Posts
-
Is Osmosis High To Low Or Low To High
Mar 15, 2025
-
Concave Mirror And Convex Mirror Difference
Mar 15, 2025
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
-
Mountain Range That Separates Europe And Asia
Mar 15, 2025
-
16 Out Of 40 As A Percentage
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Are Electrically Charged Atoms Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
