Water Boiling Is A Physical Change
News Leon
Mar 19, 2025 · 6 min read
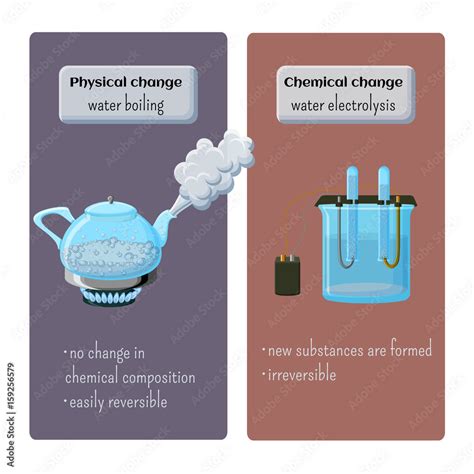
Table of Contents
- Water Boiling Is A Physical Change
- Table of Contents
- Water Boiling: A Deep Dive into a Physical Change
- Understanding Physical and Chemical Changes
- The Evidence: Why Boiling Water is a Physical Change
- 1. No New Substance is Formed
- 2. The Change is Reversible
- 3. No Change in Chemical Properties
- 4. Energy Transfer, Not Chemical Reaction
- Understanding the Process: From Liquid to Gas
- Intermolecular Forces
- Heat Energy and Kinetic Energy
- Overcoming Intermolecular Forces
- Vapor Pressure and Boiling Point
- Implications of Water Boiling: From Kitchen to Industry
- Cooking
- Industrial Processes
- Scientific Experiments and Research
- Everyday Life
- Addressing Common Misconceptions
- "Boiling creates steam, which is a different substance."
- "Boiling changes the taste of water."
- "Boiling water purifies it completely."
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Water Boiling: A Deep Dive into a Physical Change
Boiling water is a ubiquitous process, yet its fundamental nature often gets overlooked. Is it a chemical change, transforming water into something entirely different? Or is it a physical change, altering the water's form but not its composition? The answer, unequivocally, is that boiling water is a physical change. This article will delve into the science behind this seemingly simple process, exploring the concepts of physical and chemical changes, examining the evidence that supports the physical nature of boiling, and addressing common misconceptions. We'll also explore the implications of this physical change in various contexts, from cooking to industrial processes.
Understanding Physical and Chemical Changes
Before we delve into the specifics of boiling water, let's establish a clear understanding of the difference between physical and chemical changes. This distinction is crucial to understanding why boiling is classified as a physical change.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. Think of cutting paper, melting ice, or dissolving sugar in water. In each case, the substance's identity remains the same; it's merely in a different state or form. The key characteristic is that no new substance is formed. The changes are often reversible.
Chemical changes, also known as chemical reactions, involve the transformation of one or more substances into entirely new substances with different chemical properties. Burning wood, rusting iron, and baking a cake are all examples of chemical changes. These changes usually involve the breaking and forming of chemical bonds, resulting in the creation of new molecules and often irreversible changes. Key indicators of chemical change include a color change, the production of gas, the formation of a precipitate (solid), or a change in temperature.
The Evidence: Why Boiling Water is a Physical Change
Several lines of evidence strongly support the classification of boiling water as a physical change:
1. No New Substance is Formed
When water boils, it transitions from its liquid state to its gaseous state, becoming steam or water vapor. However, the chemical composition remains the same. Both liquid water and water vapor consist of H₂O molecules. There's no creation of new molecules or breaking of the oxygen-hydrogen bonds within the water molecule. This absence of new substance formation is the hallmark of a physical change.
2. The Change is Reversible
The transition from liquid water to steam is reversible. If you cool the steam, it will condense back into liquid water. This reversibility is a strong indicator of a physical change. Chemical changes are often, though not always, irreversible.
3. No Change in Chemical Properties
Boiling water doesn't alter its chemical properties. The water molecules in steam still exhibit the same characteristics as the water molecules in liquid water. For instance, the boiling point, density, and other inherent properties of water remain unchanged, even though its physical state has changed.
4. Energy Transfer, Not Chemical Reaction
The process of boiling water involves absorbing energy, primarily heat. This energy overcomes the intermolecular forces holding the water molecules together in the liquid state, allowing them to escape as gas. It's a process of energy transfer, not a chemical reaction involving the rearrangement of atoms within the molecules.
Understanding the Process: From Liquid to Gas
The boiling process is a fascinating example of how energy influences the state of matter. Let's explore the details of this transformation:
Intermolecular Forces
Water molecules are held together by relatively strong intermolecular forces, primarily hydrogen bonds. These bonds are weaker than the covalent bonds within the water molecule itself, but they are still significant enough to keep water molecules clustered together in the liquid state.
Heat Energy and Kinetic Energy
When heat is applied to water, the water molecules absorb energy, increasing their kinetic energy – the energy of their motion. This increased kinetic energy causes the molecules to move faster and vibrate more vigorously.
Overcoming Intermolecular Forces
As the kinetic energy surpasses the strength of the intermolecular forces, the water molecules can overcome their attractions to one another and escape into the gaseous phase. This is the essence of boiling. The temperature at which this occurs is the boiling point of water, which is 100°C (212°F) at standard atmospheric pressure.
Vapor Pressure and Boiling Point
The boiling point isn't a fixed value. It depends on the external pressure. At higher altitudes, where atmospheric pressure is lower, the boiling point of water is lower. Conversely, at higher pressures, the boiling point is higher. This is because the molecules need less energy to overcome the weaker intermolecular forces at lower pressures.
Implications of Water Boiling: From Kitchen to Industry
The seemingly simple physical change of boiling water has far-reaching implications in various aspects of our lives and industries.
Cooking
Boiling water is fundamental to cooking. From boiling pasta and vegetables to sterilizing utensils, it's a crucial process in food preparation. The heat transfer during boiling ensures even cooking and eliminates harmful microorganisms.
Industrial Processes
Boiling water plays a critical role in various industrial processes. It's used in power generation (steam turbines), sterilization in healthcare settings, and cleaning processes in many industries.
Scientific Experiments and Research
Boiling water is essential in numerous scientific experiments and research applications. It's used in chemistry labs for various reactions and separations, in physics for demonstrating principles of thermodynamics, and in biology for sterilization procedures.
Everyday Life
The humble act of boiling water for drinking is a critical step in ensuring safe and potable water. Boiling eliminates harmful bacteria and parasites, safeguarding our health.
Addressing Common Misconceptions
Some common misconceptions surround boiling water:
"Boiling creates steam, which is a different substance."
While steam is different in its state (gas versus liquid), it's still H₂O. The chemical composition remains the same.
"Boiling changes the taste of water."
Changes in taste after boiling are primarily due to dissolved gases escaping, not a chemical alteration of the water itself.
"Boiling water purifies it completely."
Boiling water kills many harmful microorganisms, but it doesn't remove all impurities. Filtering is usually necessary for complete purification.
Conclusion
Boiling water is a quintessential example of a physical change. The transition from liquid to gas doesn't alter the water's chemical composition; it simply changes its physical state. Understanding this distinction is crucial to appreciating the fundamental principles of chemistry and the myriad applications of this seemingly simple process in our daily lives and various industries. The reversibility of the process, the lack of new substance formation, and the consistent chemical properties throughout the change all solidify its classification as a physical change, further highlighting the importance of understanding the differences between physical and chemical transformations in the world around us.
Latest Posts
Latest Posts
-
Which Of The Following Has The Higher Energy
Mar 20, 2025
-
What Happens To A Cell In A Hypertonic Solution
Mar 20, 2025
-
The Second Phase Of Mitosis Is Called
Mar 20, 2025
-
Henrys Law Constant Co2 In Water
Mar 20, 2025
-
What Is The Most Reactive Nonmetal
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Water Boiling Is A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
