Henry's Law Constant Co2 In Water
News Leon
Mar 20, 2025 · 7 min read
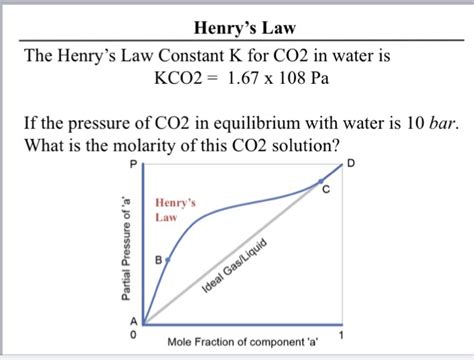
Table of Contents
Henry's Law Constant for CO2 in Water: A Deep Dive
The solubility of carbon dioxide (CO2) in water is a critical factor in numerous natural and industrial processes. Understanding this solubility is paramount in fields ranging from climate science and oceanography to carbonated beverage production and geological carbon sequestration. A key parameter governing CO2 solubility is Henry's law constant, which quantifies the relationship between the partial pressure of CO2 in the gas phase and its concentration in the aqueous phase. This article delves into the intricacies of Henry's law constant for CO2 in water, exploring its significance, influencing factors, applications, and limitations.
Understanding Henry's Law
Henry's law states that the amount of a gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid, provided the temperature remains constant. Mathematically, this is expressed as:
P = K<sub>H</sub>C
Where:
- P is the partial pressure of the gas above the liquid.
- K<sub>H</sub> is Henry's law constant.
- C is the concentration of the dissolved gas in the liquid.
The value of K<sub>H</sub> is temperature-dependent and varies with the nature of the gas and the solvent. For CO2 in water, this constant is not a simple, single number; rather, it's a complex function influenced by several factors.
Factors Affecting Henry's Law Constant for CO2 in Water
Several factors significantly influence the Henry's law constant for CO2 in water, impacting its solubility:
1. Temperature:
Temperature plays a crucial role in determining CO2 solubility. As temperature increases, the kinetic energy of CO2 molecules rises, making them more likely to escape from the aqueous phase into the gaseous phase. Consequently, the Henry's law constant for CO2 decreases with increasing temperature, signifying reduced solubility at higher temperatures. This explains why carbonated drinks go flat faster in warm conditions.
2. Pressure:
While Henry's law assumes a constant temperature, the partial pressure of CO2 directly impacts its solubility. Increasing the partial pressure of CO2 increases its solubility in water, as more CO2 molecules are forced into the liquid phase. This is the principle behind carbonating beverages under high pressure.
3. Salinity:
The presence of dissolved salts (salinity) in water can affect CO2 solubility. Generally, increasing salinity tends to decrease the solubility of CO2, a phenomenon known as salting-out. This effect is significant in marine environments where salinity influences the uptake and release of CO2 by the oceans.
4. Ionic Strength:
Similar to salinity, the overall ionic strength of the aqueous solution impacts CO2 solubility. Higher ionic strength often leads to a decrease in CO2 solubility, although the precise effect depends on the specific ions present.
5. pH:
The pH of the water is a critical factor because dissolved CO2 reacts with water to form carbonic acid (H2CO3), which then dissociates into bicarbonate (HCO3-) and carbonate (CO32-) ions. In more alkaline solutions (higher pH), the equilibrium shifts towards the formation of bicarbonate and carbonate ions, effectively increasing the overall dissolved inorganic carbon (DIC) but reducing the concentration of free CO2.
6. Presence of other dissolved gases:
The presence of other dissolved gases can influence the solubility of CO2 through competitive effects. The interaction between different gases can alter the overall behavior of the system, making the prediction of CO2 solubility more complex.
Determining Henry's Law Constant for CO2 in Water
Determining the Henry's law constant for CO2 in water requires precise experimental measurements. Common methods include:
- Gas chromatography: This technique is used to measure the amount of CO2 dissolved in water after equilibration with a known partial pressure of CO2.
- Spectroscopic methods: Techniques like infrared or Raman spectroscopy can directly measure the concentration of dissolved CO2 in water.
- Titration: This method involves chemically reacting the dissolved CO2 with a standard solution to determine its concentration.
The experimental data is then used to plot a graph of CO2 concentration versus partial pressure, and the slope of the linear portion of the graph represents the Henry's law constant at the specific temperature and conditions of the experiment.
Applications of Henry's Law Constant for CO2 in Water
The Henry's law constant for CO2 in water is crucial in various applications, including:
1. Climate Change Research:
Understanding CO2 solubility in seawater is vital for predicting the impact of anthropogenic CO2 emissions on ocean acidification and climate change. The oceans act as a significant sink for atmospheric CO2, and the rate of CO2 uptake is directly governed by Henry's law.
2. Oceanography:
Oceanographers use Henry's law to model the distribution and transport of CO2 in the marine environment, crucial for understanding marine ecosystems and predicting the effects of climate change on ocean life.
3. Carbon Capture and Storage (CCS):
CCS technologies aim to capture CO2 from industrial sources and store it underground. Predicting the long-term storage capacity requires precise knowledge of CO2 solubility in groundwater and other subsurface formations, which depends heavily on the Henry's law constant under relevant geological conditions.
4. Beverage Industry:
The beverage industry relies heavily on Henry's law for the production of carbonated drinks. Controlling the pressure and temperature during the carbonation process is crucial for achieving the desired level of carbonation.
5. Geological Carbon Sequestration:
Geological formations like depleted oil and gas reservoirs or saline aquifers are being explored as potential storage sites for CO2. Accurate prediction of CO2 behavior in these formations requires detailed understanding of its solubility under varying pressure, temperature and salinity conditions, making Henry's law constant a key parameter.
6. Environmental Monitoring:
Monitoring CO2 levels in water bodies, such as lakes and rivers, is essential for assessing water quality and environmental impact. Henry's law is used to interpret measurements of dissolved CO2 in relation to atmospheric CO2 levels.
Limitations of Henry's Law for CO2 in Water
While Henry's law provides a valuable framework for understanding CO2 solubility, it has limitations:
-
Ideal Solution Assumption: Henry's law assumes an ideal solution, where interactions between solute (CO2) and solvent (water) molecules are negligible. In reality, CO2 interacts with water to form carbonic acid and other species, deviating from ideal behavior. At high concentrations of CO2, deviations from Henry's law are significant.
-
Temperature Dependence: The temperature dependence of K<sub>H</sub> can be complex and not always accurately represented by simple equations. Precise temperature-dependent expressions for K<sub>H</sub> are often required for accurate modeling.
-
Ionic Strength Effects: The influence of ionic strength on CO2 solubility is not always straightforward and can be challenging to predict accurately.
-
Non-Equilibrium Conditions: Henry's law applies to equilibrium conditions, where the rate of CO2 entering and leaving the liquid phase is equal. In many real-world scenarios, such as dynamic ocean-atmosphere exchange, the system may not be at equilibrium.
Beyond the Simple Henry's Law: Considering Chemical Equilibrium
The simple Henry's law equation doesn't fully capture the complex chemistry of CO2 in water. CO2 reacts with water to form carbonic acid (H₂CO₃), which further dissociates into bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻) ions. Therefore, a more comprehensive model needs to consider these equilibrium reactions. These reactions are governed by equilibrium constants, and a full description of CO2 speciation in water requires a coupled system of equations involving Henry's law constant and the equilibrium constants for carbonic acid dissociation. This leads to a more accurate representation of the overall dissolved inorganic carbon (DIC) concentration in the aqueous phase.
Conclusion
Henry's law constant for CO2 in water is a fundamental parameter with broad implications across diverse scientific and industrial fields. While the simple form of Henry's law provides a useful first approximation, a comprehensive understanding requires consideration of factors like temperature, pressure, salinity, pH, and the chemical equilibrium reactions involving dissolved CO2. Accurate determination and application of Henry's law constant, along with a sophisticated understanding of the relevant chemical equilibria, are crucial for accurate modeling and prediction of CO2 behavior in various systems, particularly in addressing critical challenges like climate change mitigation and resource management. Further research continues to refine our understanding of the intricacies of CO2 solubility in water, leading to better predictive models and more effective solutions for managing this important greenhouse gas.
Latest Posts
Latest Posts
-
Naoh Acid Or Base Strong Or Weak
Mar 20, 2025
-
What Is 80 Percent Of 65
Mar 20, 2025
-
Which Of The Following Is Not Equivalent To
Mar 20, 2025
-
Time Magazine 1999 Person Of The Century
Mar 20, 2025
-
90 Inches Is How Many Yards
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Henry's Law Constant Co2 In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
