Naoh Acid Or Base Strong Or Weak
News Leon
Mar 20, 2025 · 5 min read
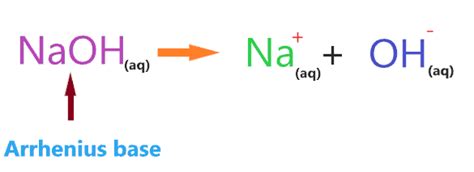
Table of Contents
NaOH: A Strong Base – Understanding its Properties and Reactions
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a strong base. Understanding its properties and how it interacts with other substances is crucial in various fields, from industrial manufacturing to everyday applications. This comprehensive guide will delve deep into the characteristics of NaOH, explaining why it's classified as a strong base and exploring its diverse reactions.
What Makes NaOH a Strong Base?
The strength of a base is determined by its ability to dissociate completely in an aqueous solution (water). Strong bases, like NaOH, readily ionize, releasing hydroxide ions (OH⁻) into the solution. This complete dissociation is what differentiates strong bases from weak bases.
The dissociation reaction of NaOH is:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This equation shows that one molecule of NaOH in water completely separates into one sodium ion (Na⁺) and one hydroxide ion (OH⁻). The presence of a high concentration of OH⁻ ions is what makes the solution highly alkaline, characterized by a high pH value (typically above 7).
Contrast with Weak Bases
Weak bases, on the other hand, only partially dissociate in water. This means that only a small fraction of the weak base molecules break down into ions, resulting in a lower concentration of OH⁻ ions and a less alkaline solution. Examples of weak bases include ammonia (NH₃) and many organic amines.
The incomplete dissociation of weak bases is represented by an equilibrium reaction, indicated by a double arrow:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
This equilibrium shows that the reaction proceeds in both directions – the formation of ammonium ions (NH₄⁺) and hydroxide ions (OH⁻) and the reverse reaction where they recombine. The equilibrium constant (Kb) for weak bases is a measure of their dissociation strength, with smaller Kb values indicating weaker bases.
Properties of NaOH
NaOH possesses several key properties that contribute to its widespread use:
-
Highly Alkaline: As a strong base, NaOH has a very high pH, typically between 13 and 14. This high alkalinity allows it to neutralize acids effectively.
-
Corrosive: NaOH is highly corrosive and can cause severe burns to skin and eyes. Appropriate safety measures, including protective eyewear, gloves, and clothing, are crucial when handling NaOH.
-
Hygroscopic: NaOH readily absorbs moisture from the air, a property known as hygroscopy. This can lead to the formation of a concentrated solution and increase the risk of accidental exposure.
-
Solubility: NaOH is highly soluble in water, readily dissolving to form an alkaline solution. Its solubility increases with temperature.
-
White Crystalline Solid: In its pure form, NaOH is a white, crystalline solid. However, commercially available NaOH is often in the form of pellets, flakes, or solutions.
Reactions of NaOH
NaOH readily participates in a wide range of chemical reactions due to its strong basicity:
1. Neutralization Reactions:
NaOH readily neutralizes acids, forming salt and water. This is a classic acid-base reaction. For example, the reaction with hydrochloric acid (HCl) is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This reaction produces sodium chloride (NaCl), common table salt, and water. Similar reactions occur with other acids, producing different salts.
2. Saponification:
NaOH plays a crucial role in the saponification process, which is the traditional method of soap making. When NaOH reacts with fats and oils (esters), it breaks them down into glycerol and fatty acid salts, which are the components of soap.
3. Ester Hydrolysis:
NaOH can also catalyze the hydrolysis of esters. In this reaction, an ester reacts with water in the presence of NaOH to form a carboxylic acid and an alcohol.
4. Reactions with Metals:
NaOH reacts with certain metals, particularly amphoteric metals like aluminum and zinc, producing hydrogen gas and a metal salt.
5. Reactions with Carbon Dioxide:
NaOH reacts with carbon dioxide (CO₂) to form sodium carbonate (Na₂CO₃) and water. This reaction is often used to absorb CO₂ in industrial processes.
Applications of NaOH
The strong basicity and reactivity of NaOH make it a valuable substance in numerous industrial and everyday applications:
-
Chemical Industry: NaOH is a vital reactant in the manufacturing of various chemicals, including soaps, detergents, paper, textiles, and many more.
-
Pulp and Paper Industry: NaOH is used in the pulping process to separate lignin from cellulose fibers in wood, a key step in paper production.
-
Food Processing: NaOH is used in various food processing applications, such as in the production of certain food additives and the neutralization of acids. It's also used in food processing equipment cleaning.
-
Drain Cleaners: Many commercially available drain cleaners contain NaOH to dissolve organic matter that causes clogs.
-
Petroleum Refining: NaOH is used in various petroleum refining processes, including the neutralization of acids and the treatment of refinery wastewater.
-
Water Treatment: NaOH is sometimes used in water treatment to adjust the pH of water, making it less acidic.
Safety Precautions when Handling NaOH
Because of its corrosive nature, handling NaOH requires strict adherence to safety procedures:
-
Protective Gear: Always wear appropriate protective gear, including safety goggles, gloves, and lab coats, when handling NaOH.
-
Ventilation: Work in a well-ventilated area to avoid inhalation of NaOH dust or fumes.
-
Spills: In case of spills, neutralize the NaOH with a dilute acid, such as vinegar, followed by thorough cleaning with water.
-
First Aid: In case of skin or eye contact, immediately flush the affected area with copious amounts of water for at least 15 minutes and seek medical attention.
Conclusion
NaOH, as a strong base, plays a crucial role in numerous industrial processes and everyday applications. Its strong basicity and reactivity enable it to participate in a wide variety of chemical reactions, including neutralization, saponification, and ester hydrolysis. However, its corrosive nature demands strict adherence to safety precautions during handling and usage. Understanding the properties and reactions of NaOH is fundamental for anyone working in fields where it is utilized, ensuring safe and efficient practices. Continued research and development will undoubtedly expand our understanding of NaOH and its potential applications further. The versatile nature of this strong base will likely ensure its continued importance in various industrial and scientific sectors for years to come. The information provided here serves as a comprehensive overview but should not replace detailed safety data sheets or professional guidance when handling this powerful chemical. Always prioritize safety and consult relevant resources before conducting any experiments or applications involving sodium hydroxide.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of Neon
Mar 21, 2025
-
A Demand Curve That Is Perfectly Inelastic Is
Mar 21, 2025
-
Molten Rock Inside The Earth Is Called
Mar 21, 2025
-
What Organelle Gets Rid Of Waste
Mar 21, 2025
-
Why Is Iron Significant To Understanding How A Supernova Occurs
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Naoh Acid Or Base Strong Or Weak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
