What Happens To A Cell In A Hypertonic Solution
News Leon
Mar 20, 2025 · 6 min read
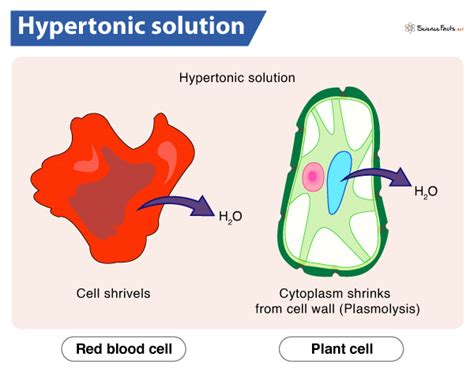
Table of Contents
What Happens to a Cell in a Hypertonic Solution? A Deep Dive into Osmosis and Cell Behavior
Understanding what happens to a cell placed in a hypertonic solution is fundamental to comprehending cellular biology and physiology. This process, driven by osmosis, significantly impacts cell structure, function, and ultimately, survival. This article delves into the intricacies of hypertonic solutions, the mechanisms of osmosis, and the diverse effects on various cell types. We'll explore the implications for health, disease, and even food preservation techniques.
Understanding Osmosis: The Driving Force
Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement aims to equalize the water potential on both sides of the membrane. The key players here are the solute (dissolved substances) and the solvent (water). The higher the solute concentration, the lower the water concentration, and vice versa.
Selective Permeability: The Gatekeeper
The selectively permeable membrane plays a crucial role. It allows water molecules to pass freely but restricts the movement of many solutes. This differential permeability is vital for maintaining cellular homeostasis. Different membranes exhibit varying degrees of selectivity, depending on their composition and the types of channels and transporters they possess. This selective permeability is what makes osmosis a directed process.
Hypertonic Solutions: A Definition
A hypertonic solution is one in which the concentration of solutes is higher outside the cell than inside the cell. This creates a water potential gradient, with a higher water potential inside the cell and a lower water potential outside. This gradient drives the movement of water.
The Impact of Solute Concentration
The degree of hypertonicity significantly impacts the outcome. A solution with a slightly higher solute concentration will have a less drastic effect compared to a solution with a drastically higher concentration. This is directly proportional; the greater the difference in solute concentration between the inside and outside of the cell, the more pronounced the effect on the cell.
What Happens to the Cell? The Process of Plasmolysis
When a cell is placed in a hypertonic solution, water moves out of the cell through osmosis, down its concentration gradient. This outward movement of water leads to several significant changes within the cell.
Shrinkage and Dehydration
The most immediate consequence is cellular shrinkage. As water leaves the cell, the cytoplasm decreases in volume, causing the cell membrane to pull away from the cell wall (in plant cells) or simply shrink (in animal cells). This process is known as plasmolysis in plant cells and crenation in animal cells. The degree of shrinkage depends on the extent of the water loss and the cell's ability to withstand the osmotic pressure.
Loss of Turgor Pressure (Plant Cells)
Plant cells rely on turgor pressure – the pressure exerted by the cytoplasm against the cell wall – to maintain their shape and rigidity. In a hypertonic solution, the loss of water reduces turgor pressure, causing the cell to become flaccid and potentially leading to wilting in the plant. This loss of turgor pressure can severely impact the plant's ability to carry out essential functions.
Cellular Damage and Dysfunction
Prolonged exposure to a hypertonic environment can lead to significant cellular damage. The dehydration can disrupt various cellular processes, affecting enzyme activity, membrane integrity, and overall cellular metabolism. Extreme cases can lead to cell death.
Disruption of Cellular Processes
Beyond structural changes, the loss of water can severely disrupt various cellular processes. Enzyme reactions, which require a specific water environment to function optimally, are hampered. Membrane transport systems, relying on proper hydration for functionality, become less efficient. These disruptions can have cascading effects throughout the cell, ultimately impacting its ability to perform its tasks.
Cell Type Specific Responses
The response of a cell to a hypertonic environment varies significantly depending on its type and structure.
Animal Cells: Crenation and Cell Death
Animal cells lack a rigid cell wall and are more vulnerable to osmotic stress. In a hypertonic solution, they undergo crenation, shrinking and potentially undergoing irreversible damage, leading to cell death.
Plant Cells: Plasmolysis and Recovery
Plant cells, with their rigid cell walls, exhibit a different response. They undergo plasmolysis, with the cell membrane pulling away from the cell wall. However, if the cell is returned to a isotonic or hypotonic environment, it can often recover its turgor pressure and resume normal function. The cell wall protects the plant cell from complete collapse unlike the animal cell.
Bacterial Cells: Plasmolysis and Resistance
Bacterial cells also display varying degrees of resistance to hypertonic environments. Some bacteria have mechanisms to counteract osmotic stress, accumulating compatible solutes to maintain cellular turgor pressure. Others may undergo plasmolysis and potentially death depending on the severity of the hypertonic condition.
Fungal Cells: Similar Responses to Plant Cells
Fungal cells, like plant cells, possess cell walls, although the composition differs. They exhibit responses similar to plant cells, undergoing plasmolysis in hypertonic solutions, with the potential for recovery under less stressful conditions.
Practical Applications and Implications
Understanding the effects of hypertonic solutions has several practical implications in various fields.
Food Preservation: Osmotic Dehydration
Hypertonic solutions are used extensively in food preservation. The process of osmotic dehydration involves placing food in a hypertonic solution, such as a high concentration of sugar or salt, leading to water loss from the food and inhibiting microbial growth. This technique is employed in preserving fruits, vegetables, and meats.
Medical Applications: Intravenous Solutions
In medicine, carefully controlled isotonic solutions are crucial for intravenous fluid therapy. Administering a hypertonic solution intravenously could cause rapid dehydration of red blood cells, leading to severe complications. Strict control of osmolarity is essential for maintaining patient health.
Environmental Considerations: Salinity and Aquatic Life
In aquatic ecosystems, variations in salinity influence the survival of aquatic organisms. Hypertonic environments, such as highly saline waters, can significantly stress aquatic life, impacting their ability to regulate water balance and survive.
Conclusion: A Critical Cellular Process
The response of a cell to a hypertonic solution is a complex interplay of osmosis, membrane permeability, and cellular structure. Understanding this process is crucial for numerous fields, including medicine, agriculture, and food science. The impact of hypertonicity ranges from reversible plasmolysis in plants to potentially fatal crenation in animal cells. The ability of cells to withstand osmotic stress is a testament to the remarkable adaptability of life. Further research continues to unravel the intricate mechanisms by which cells respond to and cope with osmotic challenges. This detailed understanding allows for the development of strategies to mitigate the negative effects of hypertonic environments in various contexts, and also provides insight into how cells regulate their internal environments and maintain essential cellular functions.
Latest Posts
Latest Posts
-
Are Frogs Omnivores Herbivores Or Carnivores
Mar 20, 2025
-
How Many Years Is A Four Score
Mar 20, 2025
-
Length Of Perpendicular From A Point To A Line
Mar 20, 2025
-
Which Muscle Type Is Striated Uninucleate And Branched
Mar 20, 2025
-
The Chemical Behavior Of An Atom Is Determined By
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Happens To A Cell In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
