True Or False Osmosis Is A Type Of Diffusion
News Leon
Mar 16, 2025 · 5 min read
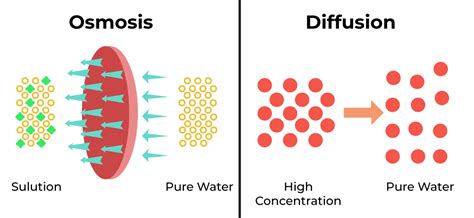
Table of Contents
True or False: Osmosis is a Type of Diffusion?
The statement "Osmosis is a type of diffusion" is true. However, understanding why this is true requires a deeper dive into the concepts of diffusion and osmosis, exploring their similarities and key differences. This article will thoroughly examine both processes, clarifying their relationship and debunking potential misconceptions.
Understanding Diffusion
Diffusion is a fundamental process in nature, describing the net movement of particles from a region of higher concentration to a region of lower concentration. This movement continues until the particles are evenly distributed throughout the available space. This passive process doesn't require energy input; it's driven by the inherent kinetic energy of the particles themselves. Think of a drop of ink spreading out in a glass of water – that's diffusion in action.
Factors Affecting Diffusion Rate
Several factors influence the rate of diffusion:
- Concentration gradient: The steeper the concentration gradient (the bigger the difference in concentration between two areas), the faster the diffusion rate. A larger difference means a stronger driving force.
- Temperature: Higher temperatures increase the kinetic energy of particles, leading to faster diffusion. Colder temperatures slow it down.
- Mass of particles: Smaller particles diffuse faster than larger ones because they move more easily.
- Surface area: A larger surface area allows for more particles to cross the boundary simultaneously, increasing the diffusion rate.
- Distance: Diffusion is faster over shorter distances. The further particles need to travel, the slower the process.
- Medium: The medium through which diffusion occurs also plays a role. Diffusion is faster in gases than in liquids, and faster in liquids than in solids.
Understanding Osmosis
Osmosis is a specialized type of diffusion. Specifically, it's the passive movement of water molecules across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. The key difference here is the presence of a selectively permeable membrane, which only allows certain substances to pass through. This membrane acts as a barrier, controlling the flow of water molecules.
Selectively Permeable Membranes: The Key to Osmosis
A selectively permeable membrane is crucial to osmosis. These membranes are often composed of lipid bilayers with embedded proteins that act as channels or carriers, regulating the passage of molecules. Water molecules can pass through these membranes relatively easily, but larger molecules or charged ions may be blocked. This selectivity is what distinguishes osmosis from simple diffusion.
Osmotic Pressure
Because osmosis is driven by a difference in water concentration, we can talk about osmotic pressure. Osmotic pressure is the pressure that would need to be applied to prevent the flow of water across a selectively permeable membrane. The higher the concentration difference, the higher the osmotic pressure.
Isotonic, Hypotonic, and Hypertonic Solutions
When discussing osmosis, understanding the terms isotonic, hypotonic, and hypertonic is crucial:
- Isotonic solution: The solute concentration is equal on both sides of the membrane. There is no net movement of water.
- Hypotonic solution: The solute concentration is lower outside the membrane than inside. Water moves into the cell, potentially causing it to swell or burst (lyse).
- Hypertonic solution: The solute concentration is higher outside the membrane than inside. Water moves out of the cell, causing it to shrink (crenate).
The Relationship Between Diffusion and Osmosis
The crucial link between diffusion and osmosis is the underlying principle of movement down a concentration gradient. In diffusion, this is the movement of any particle from high to low concentration. In osmosis, it is the specific movement of water molecules from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration) across a selectively permeable membrane.
Therefore, osmosis is a specialized form of diffusion, restricted by the presence of a selectively permeable membrane and specifically focused on the movement of water. It's not a different process entirely; it's a refined version adapted to biological systems.
Examples of Osmosis in Biological Systems
Osmosis plays a vital role in many biological processes:
- Water uptake by plant roots: Water moves from the soil (high water potential) into the roots (lower water potential) via osmosis.
- Water reabsorption in the kidneys: Water is reabsorbed from the filtrate in the nephrons back into the bloodstream through osmosis.
- Maintaining cell turgor pressure in plants: Osmosis helps maintain the firmness of plant cells, crucial for their structure and function.
- Regulation of blood pressure: Osmosis plays a part in maintaining the proper balance of fluids in the blood.
- Nutrient absorption in the intestines: Osmosis contributes to the movement of water along with nutrients into the bloodstream.
Misconceptions about Osmosis
Several misconceptions often surround osmosis:
- Osmosis only involves water: While water is the primary molecule involved, other small, uncharged molecules can sometimes move across the membrane through osmosis, though this is less common.
- Osmosis requires energy: Osmosis is a passive process; it doesn't require energy input. The movement is driven by the concentration gradient.
- Osmosis is only relevant in biological systems: Although it's crucial in biology, osmosis applies to any situation involving the movement of water across a selectively permeable membrane, including certain industrial processes.
Conclusion: Osmosis is a Specialized Type of Diffusion
In conclusion, the statement "Osmosis is a type of diffusion" is undeniably true. Osmosis is a specialized form of passive transport driven by the concentration gradient of water, specifically occurring across a selectively permeable membrane. While distinct in its focus on water and the presence of this membrane, its fundamental mechanism remains rooted in the core principles of diffusion. Understanding this relationship is crucial for comprehending various biological and chemical processes, ranging from plant growth to maintaining fluid balance in living organisms. The subtle but significant differences between diffusion and osmosis highlight the complexity and elegance of transport mechanisms in nature. By appreciating these distinctions, we can better understand the intricate interplay of forces that govern the movement of molecules and maintain the dynamic equilibrium of living systems.
Latest Posts
Latest Posts
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about True Or False Osmosis Is A Type Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
