The Substance That Dissolves The Solute
News Leon
Mar 23, 2025 · 6 min read
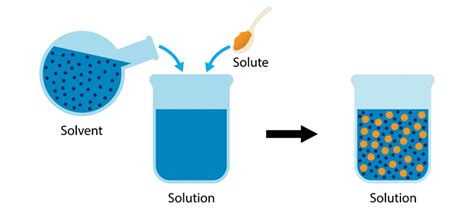
Table of Contents
The Substance That Dissolves the Solute: A Deep Dive into Solvents
The seemingly simple question, "What is the substance that dissolves the solute?" unveils a fascinating world of chemistry, encompassing various properties, interactions, and applications. The answer, of course, is the solvent. But understanding solvents goes far beyond a simple definition. This comprehensive exploration will delve into the intricacies of solvents, their properties, the types of solvents available, the factors influencing solubility, and the diverse applications of these crucial substances.
Understanding Solvents: More Than Just a Dissolver
A solvent is a substance that dissolves a solute, resulting in a homogeneous mixture called a solution. This seemingly straightforward process involves complex interactions at a molecular level. The solvent's ability to dissolve a solute depends on several crucial factors, primarily the solvent's polarity and the solute's polarity. "Like dissolves like" is a fundamental principle in chemistry that governs this interaction. Polar solvents readily dissolve polar solutes, while nonpolar solvents effectively dissolve nonpolar solutes.
Polarity: The Key to Solubility
Polarity refers to the distribution of electrical charge within a molecule. A polar molecule possesses a positive and a negative end due to an uneven distribution of electrons. Water (H₂O) is a classic example of a polar solvent, with the oxygen atom carrying a partial negative charge and the hydrogen atoms carrying partial positive charges. In contrast, nonpolar molecules have an even distribution of electrons, resulting in no significant charge separation. Examples include oils and fats.
The interaction between solvent and solute involves intermolecular forces, namely dipole-dipole interactions, hydrogen bonding, and London dispersion forces. Polar solvents, like water, utilize strong dipole-dipole interactions and hydrogen bonding to attract and surround polar solutes, effectively breaking them apart and dissolving them. Nonpolar solvents, on the other hand, rely on weaker London dispersion forces to interact with and dissolve nonpolar solutes.
Types of Solvents: A Diverse Range of Substances
Solvents encompass a vast array of chemical substances, each with its unique properties and applications. Broadly, they can be categorized as follows:
1. Polar Protic Solvents
These solvents are polar and contain hydrogen atoms bonded to highly electronegative atoms like oxygen or nitrogen. This allows them to form strong hydrogen bonds, making them excellent solvents for polar and ionic compounds. Examples include:
- Water (H₂O): The most common and versatile solvent, essential for biological processes and countless industrial applications.
- Methanol (CH₃OH): A highly polar and readily miscible solvent used in various industrial processes and as a fuel additive.
- Ethanol (CH₃CH₂OH): Widely used as a solvent in pharmaceuticals, cosmetics, and beverages.
- Acetic Acid (CH₃COOH): A polar protic solvent with unique properties, employed in the production of polymers and pharmaceuticals.
2. Polar Aprotic Solvents
These solvents are polar but lack an O-H or N-H bond, preventing them from forming hydrogen bonds. They are often good solvents for ionic compounds and some polar organic molecules. Examples include:
- Acetone (CH₃COCH₃): A widely used solvent in various industries, known for its excellent dissolving capabilities.
- Dimethyl Sulfoxide (DMSO): A powerful solvent often used in organic chemistry and as a carrier for pharmaceuticals.
- Dimethylformamide (DMF): Another common solvent utilized in organic chemistry and various industrial processes.
- Acetonitrile (CH₃CN): Often used as a solvent in electrochemistry and high-performance liquid chromatography (HPLC).
3. Nonpolar Solvents
These solvents lack significant polarity and primarily rely on London dispersion forces to dissolve nonpolar solutes. They are often used to dissolve fats, oils, and other nonpolar substances. Examples include:
- Hexane (C₆H₁₄): A common nonpolar solvent used in the extraction of oils and fats.
- Benzene (C₆H₆): A powerful solvent with carcinogenic properties, its use is now restricted.
- Toluene (C₇H₈): Often used as a solvent in paints, thinners, and adhesives.
- Diethyl Ether (CH₃CH₂OCH₂CH₃): A volatile and flammable solvent previously used extensively in organic chemistry.
Factors Influencing Solubility: Beyond Polarity
While polarity plays a crucial role in solubility, other factors also significantly influence a solvent's ability to dissolve a solute:
- Temperature: Increasing temperature generally increases the solubility of solids and liquids in liquids. However, the effect on gas solubility is the opposite; increased temperature decreases gas solubility.
- Pressure: Pressure significantly affects the solubility of gases in liquids. Higher pressure increases the solubility of gases. The effect of pressure on the solubility of solids and liquids is minimal.
- Particle Size: Smaller solute particles dissolve faster than larger ones due to the increased surface area exposed to the solvent.
- Stirring/Agitation: Stirring or agitating the solution accelerates the dissolving process by constantly bringing fresh solvent into contact with the solute.
Applications of Solvents: A Wide Range of Industries
Solvents are ubiquitous, finding applications across numerous industries:
1. Pharmaceutical Industry:
Solvents play a critical role in the formulation and manufacturing of pharmaceuticals, enabling the dissolution of active ingredients and the production of various dosage forms like tablets, capsules, and injectables.
2. Chemical Industry:
Solvents are indispensable in chemical reactions, acting as reaction media, facilitating the mixing of reactants, and controlling reaction rates and selectivity.
3. Coatings and Paints:
Solvents are crucial components of paints and coatings, dissolving the resin and pigment, controlling viscosity, and affecting the drying process.
4. Cleaning Industry:
Solvents are essential in cleaning agents, removing grease, dirt, and other contaminants from various surfaces.
5. Food and Beverage Industry:
Specific solvents are used in food processing and extraction to isolate valuable components like flavors, aromas, and natural pigments.
6. Electronics Industry:
Solvents play a significant role in the manufacturing of electronic components, cleaning and degreasing parts and assisting in the deposition of thin films.
Green Solvents and Sustainability: The Future of Solvents
Concerns about the environmental impact of traditional solvents have spurred research into green solvents, which are less toxic, biodegradable, and derived from renewable sources. Examples include supercritical carbon dioxide (scCO₂), ionic liquids, and deep eutectic solvents. The transition to greener solvent options is vital for sustainable development and reducing the environmental footprint of various industries.
Conclusion: The Solvent's Vital Role
The substance that dissolves the solute – the solvent – is far more than a simple component of a solution. Understanding its properties, types, and interactions is critical across numerous scientific disciplines and industries. The choice of solvent significantly influences the outcome of chemical processes, the efficiency of industrial operations, and the overall sustainability of various applications. From the ubiquitous water to the specialized green solvents of the future, the world of solvents continues to evolve, driving innovation and progress across diverse fields. Further research and development in this area will undoubtedly lead to the discovery of novel solvents with improved properties and reduced environmental impact, furthering our understanding of this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
What Is A Result Of Drinking Too Much Alcohol Milady
Mar 24, 2025
-
Why Is Blood Considered A Connective Tissue
Mar 24, 2025
-
Which Of The Following Is A Colligative Property
Mar 24, 2025
-
What Is Not True About The Brain
Mar 24, 2025
-
Which Of The Following Pairs Are Incorrectly Matched
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about The Substance That Dissolves The Solute . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
