Which Of The Following Is A Colligative Property
News Leon
Mar 24, 2025 · 6 min read
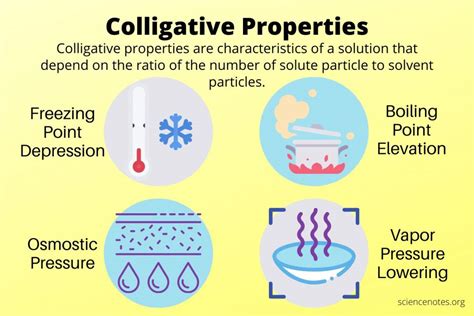
Table of Contents
Which of the Following is a Colligative Property? Understanding Colligative Properties and Their Applications
Colligative properties are a fascinating aspect of chemistry, impacting numerous applications in our daily lives. Understanding these properties is crucial for anyone studying chemistry, from introductory students to seasoned researchers. This in-depth article will explore colligative properties, delve into the four main types, explain their underlying principles, and illustrate their practical applications. We'll also address common misconceptions and provide examples to solidify your understanding. By the end, you'll be able to confidently identify colligative properties and understand their significance.
What are Colligative Properties?
Colligative properties are properties of solutions that depend on the concentration of solute particles, not their identity. This means that the property is affected by the number of particles present, regardless of what those particles actually are. This contrasts with other solution properties, such as color or viscosity, which are heavily influenced by the specific chemical nature of the solute. The key is that colligative properties are determined by the number of particles, not their type.
This seemingly simple distinction has profound implications. It allows us to predict the behavior of solutions based solely on the concentration of dissolved particles, simplifying complex systems and enabling powerful analytical techniques.
The Four Main Colligative Properties
There are four primary colligative properties:
1. Vapor Pressure Lowering
When a non-volatile solute is added to a solvent, the vapor pressure of the resulting solution is lower than the vapor pressure of the pure solvent. This is because the solute particles occupy some of the surface area of the liquid, reducing the number of solvent molecules that can escape into the gaseous phase. The extent of vapor pressure lowering is directly proportional to the mole fraction of the solute.
Raoult's Law quantitatively describes this phenomenon:
P<sub>solution</sub> = X<sub>solvent</sub> * P<sup>0</sup><sub>solvent</sub>
Where:
- P<sub>solution</sub> is the vapor pressure of the solution
- X<sub>solvent</sub> is the mole fraction of the solvent
- P<sup>0</sup><sub>solvent</sub> is the vapor pressure of the pure solvent
This law is particularly useful for ideal solutions, where solute-solvent interactions are similar to solute-solute and solvent-solvent interactions. Deviations from Raoult's Law are observed in non-ideal solutions.
2. Boiling Point Elevation
Adding a non-volatile solute to a solvent increases its boiling point. This happens because the vapor pressure of the solution is lower than that of the pure solvent (as discussed above). To achieve boiling, the vapor pressure must equal the atmospheric pressure. Since the vapor pressure is lowered, a higher temperature is required to reach this equilibrium.
The elevation in boiling point (ΔT<sub>b</sub>) is directly proportional to the molality (m) of the solute:
ΔT<sub>b</sub> = K<sub>b</sub> * m
Where:
- K<sub>b</sub> is the ebullioscopic constant (a property of the solvent)
- m is the molality of the solute (moles of solute per kilogram of solvent)
3. Freezing Point Depression
Conversely, adding a solute to a solvent lowers its freezing point. This occurs because the solute particles interfere with the formation of the solvent's crystal lattice, making it more difficult for the solvent to solidify. Similar to boiling point elevation, the freezing point depression (ΔT<sub>f</sub>) is proportional to the molality of the solute:
ΔT<sub>f</sub> = K<sub>f</sub> * m
Where:
- K<sub>f</sub> is the cryoscopic constant (a property of the solvent)
- m is the molality of the solute
This principle is used in antifreeze solutions, where adding substances like ethylene glycol lowers the freezing point of water, preventing it from freezing in cold temperatures.
4. Osmotic Pressure
Osmosis is the movement of solvent molecules across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. Osmotic pressure is the pressure required to prevent osmosis from occurring. It is directly proportional to the molarity (M) of the solute and the absolute temperature (T):
π = MRT
Where:
- π is the osmotic pressure
- M is the molarity of the solute (moles of solute per liter of solution)
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
Osmotic pressure plays a vital role in biological systems, regulating the movement of water and nutrients across cell membranes.
Identifying Colligative Properties: Examples and Non-Examples
To firmly grasp the concept, let's examine several examples and non-examples:
Examples of Colligative Properties:
- The addition of salt to water raises its boiling point. The increased concentration of solute particles elevates the boiling point.
- Antifreeze in a car radiator lowers the freezing point of water. This classic example utilizes the freezing point depression property.
- Seawater has a lower vapor pressure than pure water. The dissolved salts in seawater lower its vapor pressure.
- Water moves into a cell placed in a hypotonic solution. Osmosis drives water into the cell due to the difference in solute concentration.
Non-Examples of Colligative Properties:
- The color of a solution. The color depends entirely on the chemical nature of the solute, not just its concentration.
- The viscosity of a solution. Viscosity is influenced by the size and shape of solute molecules, not just their number.
- The density of a solution. Density is affected by the mass and volume of both solute and solvent.
- The acidity (pH) of a solution. pH is dependent on the chemical nature of the solute, specifically its ability to donate or accept protons.
Applications of Colligative Properties
Colligative properties have numerous practical applications across various fields:
- Antifreeze: Used in automobiles and other systems to prevent freezing in cold climates.
- De-icing agents: Used on roads and runways to melt ice and snow.
- Desalination: Techniques like reverse osmosis use osmotic pressure to remove salts from seawater.
- Food preservation: Adding salt or sugar to food lowers the water activity, inhibiting microbial growth.
- Medical applications: Intravenous solutions must have an appropriate osmotic pressure to avoid damaging cells.
- Industrial processes: Colligative properties are important in many industrial chemical processes, such as distillation and crystallization.
Advanced Concepts and Considerations
While we have focused on ideal solutions, it's crucial to note that real-world solutions often deviate from ideal behavior. Strong solute-solvent interactions, as well as ion pairing in electrolyte solutions, can significantly influence colligative properties. These deviations are often accounted for using activity coefficients, which correct for non-ideality.
Conclusion: Mastering the Concept of Colligative Properties
Colligative properties are a fundamental concept in chemistry with far-reaching implications. Understanding these properties – vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure – is crucial for comprehending the behavior of solutions and applying this knowledge to diverse real-world scenarios. By focusing on the number of solute particles rather than their identity, we can make accurate predictions and design effective applications. Remember that while ideal solutions provide a simplified model, recognizing the limitations and considering deviations from ideality allows for a more nuanced and complete understanding of solution behavior. Mastering these concepts is essential for anyone seeking a robust understanding of chemistry.
Latest Posts
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Colligative Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.