The Ph Of A Solution Is Defined As
News Leon
Mar 15, 2025 · 6 min read
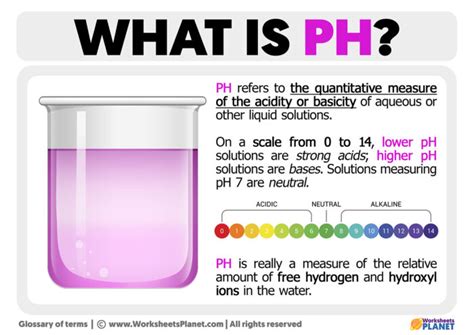
Table of Contents
The pH of a Solution: A Comprehensive Guide
The pH of a solution is a fundamental concept in chemistry, biology, and numerous other fields. Understanding pH is crucial for comprehending a wide range of processes, from the acidity of rain to the functioning of biological systems. This comprehensive guide delves into the definition, calculation, measurement, and significance of pH, offering a detailed exploration for both beginners and those seeking a deeper understanding.
Defining pH: A Measure of Acidity and Alkalinity
The pH of a solution is defined as the negative logarithm (base 10) of the hydrogen ion concentration ([H⁺]). Mathematically, it's represented as:
pH = -log₁₀[H⁺]
This seemingly simple equation holds immense significance. A higher concentration of hydrogen ions ([H⁺]) leads to a lower pH value, indicating a more acidic solution. Conversely, a lower concentration of hydrogen ions results in a higher pH value, indicating a more alkaline (or basic) solution.
The pH scale typically ranges from 0 to 14, although solutions with pH values below 0 or above 14 are possible under specific conditions. A pH of 7 is considered neutral, meaning the concentration of hydrogen ions equals the concentration of hydroxide ions (OH⁻). Solutions with a pH below 7 are acidic, and those with a pH above 7 are alkaline or basic.
Understanding the Logarithmic Scale
It's crucial to grasp the logarithmic nature of the pH scale. Each whole number change in pH represents a tenfold change in hydrogen ion concentration. For example:
- A solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
- A solution with a pH of 1 is one hundred times more acidic than a solution with a pH of 3 (10 x 10 = 100).
This logarithmic scale allows for a convenient representation of a wide range of hydrogen ion concentrations, from extremely acidic to extremely alkaline.
Calculating pH: From Concentration to Value
Calculating the pH of a solution requires knowing the concentration of hydrogen ions ([H⁺]). This concentration is typically expressed in moles per liter (mol/L) or molarity (M). Let's explore some examples:
Example 1: Strong Acid
Suppose we have a 0.01 M solution of hydrochloric acid (HCl), a strong acid that completely dissociates in water:
HCl → H⁺ + Cl⁻
In this case, the concentration of H⁺ is equal to the concentration of HCl, which is 0.01 M. Therefore:
pH = -log₁₀(0.01) = 2
The pH of the 0.01 M HCl solution is 2.
Example 2: Weak Acid
Weak acids, unlike strong acids, do not fully dissociate in water. Their dissociation is described by an equilibrium constant, Ka (acid dissociation constant). Calculating the pH of a weak acid solution requires considering this equilibrium. The calculation often involves the quadratic formula or iterative methods.
Example 3: Strong Base
For strong bases, like sodium hydroxide (NaOH), which completely dissociates, the calculation involves finding the pOH (negative logarithm of hydroxide ion concentration) first and then using the relationship:
pH + pOH = 14
Example 4: Weak Base
Similar to weak acids, calculating the pH of a weak base solution involves considering the equilibrium constant, Kb (base dissociation constant).
Measuring pH: Practical Applications
While calculating pH is important, practical applications often rely on direct measurement. Several methods exist for determining the pH of a solution:
1. pH Indicators: Visual Estimation
pH indicators are substances that change color depending on the pH of the solution. These indicators are often used in titrations to determine the endpoint of an acid-base reaction. Litmus paper, a common pH indicator, turns red in acidic solutions and blue in alkaline solutions. More sophisticated indicators provide a broader range of color changes across a wider pH spectrum. However, pH indicators offer only an approximate measurement.
2. pH Meters: Precise Measurement
pH meters are electronic instruments that provide a precise measurement of pH. They typically consist of a pH-sensitive electrode (glass electrode) and a reference electrode. The potential difference between these electrodes is measured and converted to a pH value. pH meters are widely used in various applications requiring accurate pH determination, such as in laboratories, environmental monitoring, and industrial processes. Calibration with standard buffer solutions is essential for accurate measurements.
3. Spectrophotometry: Advanced Techniques
Spectrophotometry can be used to determine pH by measuring the absorbance of a solution at specific wavelengths. Certain substances exhibit changes in their absorbance spectra depending on the pH of the solution, allowing for indirect pH determination.
The Significance of pH: Impact Across Disciplines
The pH of a solution plays a crucial role in numerous areas:
1. Biology and Medicine: Maintaining Homeostasis
Maintaining the correct pH is essential for the proper functioning of biological systems. The pH of blood, for instance, is tightly regulated around 7.4. Deviations from this value can have serious health consequences. Enzymes, the catalysts of biological reactions, have optimal pH ranges for their activity. Changes in pH can alter enzyme structure and function, affecting metabolic processes. In medicine, pH measurements are used in various diagnostic tests and treatments.
2. Environmental Science: Water Quality and Acid Rain
The pH of water bodies is a crucial indicator of water quality. Acid rain, caused by atmospheric pollutants, lowers the pH of lakes and rivers, harming aquatic life. Monitoring and regulating the pH of water is essential for protecting ecosystems.
3. Agriculture: Soil pH and Plant Growth
The pH of soil significantly impacts plant growth. Different plants thrive within specific pH ranges. Testing soil pH and adjusting it through amendments is a crucial aspect of successful agriculture.
4. Industry: Chemical Processes and Food Production
Many industrial processes require precise control of pH. In chemical manufacturing, pH adjustments are critical for ensuring the efficiency and safety of reactions. In the food industry, pH control is crucial for food preservation, safety, and quality.
5. Chemistry: Acid-Base Reactions and Equilibria
pH is a fundamental concept in acid-base chemistry. Understanding pH is essential for comprehending acid-base reactions, equilibria, and titrations.
Conclusion: pH - A Multifaceted Concept
The pH of a solution, though defined by a simple equation, has far-reaching implications across various disciplines. Its significance in biology, medicine, environmental science, agriculture, and industry underscores the importance of understanding this fundamental concept. Whether through calculation, measurement, or a combination of both, determining and controlling pH is critical for numerous processes and applications. This comprehensive guide provides a foundational understanding of pH, equipping readers with the knowledge to appreciate its importance and applications in the world around us. Further exploration into specific applications can lead to a deeper appreciation of the multifaceted role of pH in shaping our world.
Latest Posts
Latest Posts
-
Mountain Range That Separates Europe And Asia
Mar 15, 2025
-
16 Out Of 40 As A Percentage
Mar 15, 2025
-
Which Of The Following Is A True Solution
Mar 15, 2025
-
How Many Vertices Does A Rectangular Pyramid Have
Mar 15, 2025
-
Which Are Different Forms Of The Same Gene
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about The Ph Of A Solution Is Defined As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
