Which Of The Following Is A True Solution
News Leon
Mar 15, 2025 · 6 min read
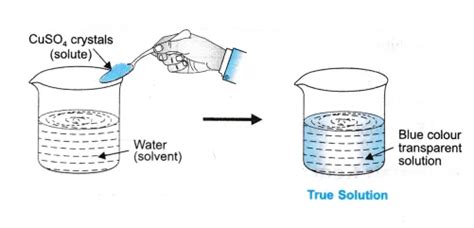
Table of Contents
Which of the Following is a True Solution? Understanding Solution Types in Chemistry
The question, "Which of the following is a true solution?" often appears in chemistry exams and requires a solid understanding of solution chemistry. This isn't simply about memorizing definitions; it's about grasping the fundamental principles that differentiate true solutions from other types of mixtures. This article will delve deep into the topic, explaining the characteristics of true solutions and contrasting them with colloids and suspensions. We'll explore particle size, filterability, and the Tyndall effect, providing examples to solidify your understanding.
What is a Solution?
A solution is a homogeneous mixture composed of two or more substances. Crucially, it's homogeneous, meaning the components are uniformly distributed throughout the mixture at a molecular level. You won't be able to visually distinguish the different components; they're intimately mixed. This homogeneity is a key characteristic that sets true solutions apart from other mixtures.
This uniformity extends to the solution's properties. A true solution will have uniform properties throughout – the concentration of the dissolved substance (the solute) is the same everywhere in the solution. This contrasts with heterogeneous mixtures where different parts of the mixture have different properties.
Key Components of a Solution:
-
Solute: This is the substance that dissolves in another substance. It's usually present in a smaller amount than the solvent. Examples include salt (NaCl), sugar (sucrose), and many other solids, liquids, or gases.
-
Solvent: This is the substance that dissolves the solute. It's usually present in a larger amount. Water is the most common solvent, but many others exist, including ethanol, acetone, and benzene.
Types of Solutions:
Solutions can be classified based on the states of matter of the solute and solvent:
- Solid in liquid: Saltwater (NaCl in water)
- Liquid in liquid: Alcohol in water
- Gas in liquid: Carbon dioxide in water (carbonated water)
- Solid in solid: Alloys (e.g., brass – a mixture of copper and zinc)
- Gas in gas: Air (a mixture of nitrogen, oxygen, and other gases)
- Liquid in gas: Water vapor in air (humidity)
Differentiating True Solutions from Colloids and Suspensions
The key to determining if a mixture is a true solution lies in comparing its properties to those of colloids and suspensions. These are also mixtures, but they differ significantly in terms of particle size and other characteristics.
1. Particle Size: The Defining Factor
The primary distinction between these three types of mixtures lies in the size of the dispersed particles:
-
True Solutions: Particle size is extremely small, typically less than 1 nanometer (nm). The solute particles are individual ions or molecules completely dispersed and dissolved within the solvent.
-
Colloids: Particle size ranges from 1 nm to 1000 nm (1 micrometer). These particles are larger than those in true solutions but still small enough to remain suspended indefinitely without settling. Examples include milk, fog, and gelatin.
-
Suspensions: Particle size is larger than 1000 nm. The particles in suspensions are large enough to settle out of the mixture over time if left undisturbed. Examples include muddy water, sand in water, and blood.
2. Filterability: Separating the Components
Another way to distinguish between these mixtures is through filtration:
-
True Solutions: The solute particles are too small to be filtered out. You can't separate the solute from the solvent using a typical filter paper.
-
Colloids: Colloidal particles are small enough to pass through ordinary filter paper. However, they can be separated using specialized techniques like ultrafiltration or dialysis.
-
Suspensions: The particles in suspensions are large enough to be easily separated from the solvent using ordinary filtration.
3. The Tyndall Effect: Scattering Light
The Tyndall effect is a phenomenon where a light beam passing through a mixture is scattered by the particles. This effect is readily visible in colloids and suspensions but not in true solutions:
-
True Solutions: Do not exhibit the Tyndall effect. The light passes straight through without scattering.
-
Colloids: Show the Tyndall effect. The light beam is scattered as it passes through, creating a visible cone of light.
-
Suspensions: Also show the Tyndall effect, even more prominently than colloids, due to the larger particle size.
Examples to Clarify the Differences
Let's illustrate these concepts with specific examples:
Scenario 1: You dissolve sugar in water. The sugar completely dissolves, forming a homogeneous mixture. The sugar molecules are dispersed at the molecular level. You cannot filter out the sugar, and a light beam passes straight through without scattering. This is a true solution.
Scenario 2: You mix milk and water. The milk particles are suspended in the water, creating a cloudy appearance. Although you might not see the individual particles with the naked eye, they are much larger than individual molecules. The mixture scatters light (Tyndall effect), and the particles won't settle out easily. This is a colloid.
Scenario 3: You mix sand and water. The sand particles are clearly visible and will settle at the bottom of the container over time. The mixture is heterogeneous, and you can easily filter out the sand. The mixture strongly scatters light (Tyndall effect). This is a suspension.
Factors Affecting Solubility in True Solutions
Several factors influence how well a solute dissolves in a solvent to form a true solution:
-
Nature of the solute and solvent: "Like dissolves like" is a common rule. Polar solvents (like water) tend to dissolve polar solutes, while nonpolar solvents (like oil) dissolve nonpolar solutes.
-
Temperature: Increasing the temperature usually increases the solubility of solids and gases in liquids. However, the effect on gases can be more complex.
-
Pressure: Pressure has a significant effect on the solubility of gases in liquids. Increasing pressure increases the solubility of gases.
Applications of True Solutions
True solutions are ubiquitous in everyday life and are crucial in various scientific and industrial processes. Here are a few examples:
-
Pharmaceuticals: Many medicines are administered as true solutions for better absorption and bioavailability.
-
Agriculture: Fertilizers are often applied as solutions to ensure uniform distribution of nutrients.
-
Food industry: Many food products, such as soft drinks and juices, are true solutions.
-
Chemical reactions: Many chemical reactions take place in solution, allowing for efficient mixing and interaction of reactants.
Conclusion: Identifying True Solutions
Determining if a mixture is a true solution involves considering several factors: the size of the dispersed particles (less than 1 nm), the ability to filter the components (unfilterable), the presence or absence of the Tyndall effect (absent), and the homogeneity of the mixture. By understanding these characteristics, you can confidently distinguish true solutions from colloids and suspensions and answer the question, "Which of the following is a true solution?" with accuracy. Remember, a thorough understanding of the fundamental principles of solution chemistry is key to mastering this important concept.
Latest Posts
Latest Posts
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A True Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
