The Function Of The Enzyme Atp Synthase Is To
News Leon
Apr 05, 2025 · 5 min read
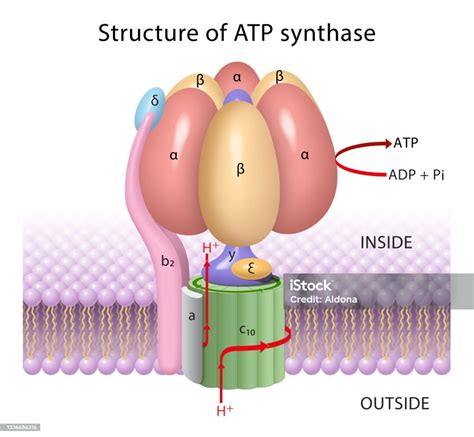
Table of Contents
The Function of the Enzyme ATP Synthase Is To… Power Life Itself!
ATP synthase, a remarkable molecular machine, sits at the heart of cellular energy production. Its primary function, as its name suggests, is to synthesize adenosine triphosphate (ATP), the cell's primary energy currency. But understanding this seemingly simple statement requires delving into the intricate mechanisms and profound implications of this crucial enzyme. This article will explore the multifaceted roles of ATP synthase, its structure, its mechanism of action, its regulation, and its significance across diverse biological systems.
The Central Role of ATP in Cellular Processes
Before diving into the intricacies of ATP synthase, let's briefly appreciate the fundamental role ATP plays in maintaining life. ATP, a nucleotide composed of adenine, ribose, and three phosphate groups, is the primary energy carrier in all living organisms. The energy stored in the high-energy phosphate bonds fuels countless cellular processes, including:
- Muscle contraction: The shortening of muscle fibers, enabling movement, relies heavily on ATP hydrolysis.
- Active transport: Moving molecules against their concentration gradient, essential for maintaining cellular homeostasis, requires ATP.
- Biosynthesis: Building complex molecules like proteins, nucleic acids, and lipids from simpler precursors consumes considerable ATP.
- Signal transduction: Cellular communication processes often involve ATP-dependent phosphorylation cascades.
- DNA replication and repair: These vital processes for maintaining genetic integrity require ATP for numerous enzymatic steps.
- Cell division: The complex processes of mitosis and meiosis necessitate substantial ATP expenditure.
The Structure of ATP Synthase: A Rotational Motor
ATP synthase is a remarkable example of biological nanotechnology, exhibiting stunning elegance in its structure and function. This enzyme is a multi-subunit complex, broadly categorized into two main domains:
-
F<sub>0</sub> (or c-ring): This hydrophobic domain is embedded within the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). It acts as a proton channel, allowing the passage of protons (H<sup>+</sup>) across the membrane. The core of F<sub>0</sub> comprises a ring of 'c' subunits, the number of which varies depending on the organism. This ring rotates as protons flow through it.
-
F<sub>1</sub> (or α<sub>3</sub>β<sub>3</sub>γδε): This hydrophilic domain protrudes into the mitochondrial matrix (or cytoplasm) and is responsible for ATP synthesis. It consists of three α and three β subunits arranged alternately, forming a hexameric structure. Only the β subunits possess the catalytic sites for ATP synthesis. The γ, δ, and ε subunits form a central stalk that connects F<sub>1</sub> to F<sub>0</sub> and acts as a rotor.
The Rotary Mechanism: A Symphony of Protons and Conformational Changes
The function of ATP synthase is intrinsically linked to its unique rotary mechanism. This elegantly designed system harnesses the proton motive force (PMF)—a gradient of protons across the membrane—to drive ATP synthesis. Here's how it works:
-
Proton Flow: Protons, accumulated in the intermembrane space (in mitochondria) or outside the cell (in prokaryotes) due to the electron transport chain, flow down their concentration gradient through the F<sub>0</sub> domain.
-
Rotation of the c-ring: The proton flow causes the c-ring to rotate. This rotation is facilitated by conformational changes within the c subunits as they bind and release protons.
-
Rotation of the γ subunit: The rotating c-ring drives the rotation of the central stalk, including the γ subunit.
-
Conformational Changes in β subunits: The rotation of the γ subunit induces conformational changes in the three β subunits, cycling them through three distinct states:
- Loose (L): ATP is loosely bound.
- Tight (T): ATP is synthesized.
- Open (O): ATP is released.
-
ATP Synthesis: As the β subunits cycle through these states, ADP and inorganic phosphate (Pi) are bound, ATP is synthesized, and then released, resulting in a net production of ATP.
Regulation of ATP Synthase: Balancing Supply and Demand
The activity of ATP synthase isn't a constant, unregulated process. Cells must carefully regulate ATP synthesis to match the energy demands of various cellular processes. Several mechanisms control ATP synthase activity:
-
Proton Motive Force: The PMF itself serves as a primary regulator. A higher PMF accelerates ATP synthesis, whereas a lower PMF slows it down.
-
Inhibitors: Certain molecules, such as oligomycin, can specifically inhibit ATP synthase by blocking proton flow through the F<sub>0</sub> domain.
-
Allosteric Regulation: The levels of ADP, ATP, and Pi can influence the enzyme's activity through allosteric interactions. High ATP levels tend to inhibit ATP synthase, while high ADP and Pi levels stimulate it.
-
Feedback Inhibition: The overall energy charge of the cell (the ratio of ATP to ADP and AMP) influences ATP synthase activity, ensuring efficient energy management.
ATP Synthase Across Biological Kingdoms
While the basic principles of ATP synthase function remain conserved across different organisms, variations exist in its structure and regulatory mechanisms.
-
Mitochondria (Eukaryotes): Mitochondrial ATP synthase plays a central role in oxidative phosphorylation, the major pathway for ATP generation in eukaryotic cells.
-
Chloroplasts (Eukaryotes): Chloroplasts utilize a similar ATP synthase to generate ATP during photosynthesis, harnessing the proton gradient created by the light-dependent reactions.
-
Bacteria and Archaea (Prokaryotes): Prokaryotic ATP synthase is found in the plasma membrane and participates in various energy-generating processes, including respiration and fermentation.
ATP Synthase and Human Health: Implications of Dysfunction
Given the crucial role ATP synthase plays in energy metabolism, its malfunction can have significant consequences for human health. Mutations in ATP synthase genes can lead to a variety of disorders, including:
-
Mitochondrial myopathies: These conditions affect muscle function due to impaired ATP production in mitochondria.
-
Neurodegenerative diseases: Defects in ATP synthase can contribute to neuronal dysfunction and neurodegenerative processes.
-
Developmental disorders: ATP synthase dysfunction can impair cellular development and lead to various congenital abnormalities.
Conclusion: A Molecular Marvel of Energy Conversion
ATP synthase stands as a testament to the elegance and efficiency of biological systems. Its intricate structure and rotary mechanism beautifully illustrate the power of natural selection to create highly optimized molecular machines. This enzyme's function—the synthesis of ATP, the cell's universal energy currency—is fundamental to all life, and its dysfunction has profound implications for human health. Further research into ATP synthase's structure, regulation, and function continues to unravel new insights into cellular energy metabolism and provides potential avenues for therapeutic interventions in various diseases. The study of this remarkable enzyme offers a deep appreciation for the intricacies of life itself.
Latest Posts
Latest Posts
-
Which Of The Following Is An Isoelectronic Series
Apr 05, 2025
-
The Reactions Of Glycolysis Occur In The
Apr 05, 2025
-
The Net Gain Of Energy From Glycolysis Is
Apr 05, 2025
-
Which Of The Following Is An Assumption Of Theory Y
Apr 05, 2025
-
Write The Electronic Configuration Of Sodium
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about The Function Of The Enzyme Atp Synthase Is To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
