The Final Electron Acceptor In Aerobic Respiration Is
News Leon
Mar 21, 2025 · 5 min read
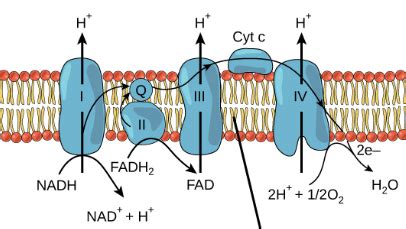
Table of Contents
The Final Electron Acceptor in Aerobic Respiration Is… Oxygen! Understanding the Crucial Role of Oxygen in Cellular Energy Production
Aerobic respiration, the process that powers most life on Earth, relies on a crucial final step: the acceptance of electrons. This seemingly simple act is the culmination of a complex chain of events, and understanding its significance is key to grasping the intricacies of cellular energy production. This article delves deep into the role of oxygen as the final electron acceptor in aerobic respiration, exploring its mechanism, importance, and the consequences of its absence.
The Electron Transport Chain: A Cascade of Energy Transfer
Before we focus on the final electron acceptor, it's essential to understand the electron transport chain (ETC), the central pathway where electrons are passed from one molecule to another. This chain, located within the inner mitochondrial membrane in eukaryotes and the plasma membrane in prokaryotes, is composed of a series of protein complexes (Complexes I-IV) and mobile electron carriers like ubiquinone (CoQ) and cytochrome c.
How the ETC Works: A Step-by-Step Breakdown
-
NADH and FADH2 Delivery: The ETC begins with the delivery of high-energy electrons from NADH and FADH2, molecules produced during glycolysis and the Krebs cycle. These molecules act as electron donors, carrying electrons harvested from the breakdown of glucose.
-
Electron Transfer Through Complexes: The electrons are then passed along the ETC, moving from one protein complex to another. Each transfer releases energy, which is harnessed to pump protons (H+) across the inner mitochondrial membrane, creating a proton gradient.
-
Proton Gradient and Chemiosmosis: This proton gradient, with a higher concentration of protons in the intermembrane space, stores potential energy. This energy is then used to drive ATP synthesis through chemiosmosis, where protons flow back across the membrane through ATP synthase, an enzyme that produces ATP (adenosine triphosphate), the cell's primary energy currency.
-
Oxygen's Crucial Role: The final step involves the transfer of electrons to the final electron acceptor, oxygen (O2). This process is crucial because it prevents the ETC from becoming "backed up." Without a final acceptor, the electron flow would cease, halting ATP production.
Oxygen: The Ultimate Electron Hog
Oxygen's high electronegativity makes it the ideal final electron acceptor. Its strong affinity for electrons allows it to readily accept the electrons passed down the ETC. This acceptance is not a passive process; it's the driving force that makes the entire system work. Without oxygen, the ETC would quickly become saturated, and ATP production would grind to a halt.
The Reduction of Oxygen: Forming Water
When oxygen accepts the electrons, it's reduced, meaning it gains electrons. This reduction reaction combines oxygen with protons (H+) to form water (H2O), a crucial byproduct of aerobic respiration. This reaction completes the electron transport chain and ensures the continuous flow of electrons.
The reaction is as follows:
4e⁻ + 4H⁺ + O₂ → 2H₂O
This simple equation beautifully encapsulates the significance of oxygen in respiration. It's the final step that allows for the efficient extraction of energy from glucose, a process vital for cellular function.
Consequences of Oxygen Absence: Anaerobic Respiration and Fermentation
When oxygen is unavailable, organisms must resort to alternative metabolic pathways to generate energy. These pathways, known as anaerobic respiration and fermentation, are far less efficient than aerobic respiration.
Anaerobic Respiration: Using Alternative Electron Acceptors
Some organisms can utilize alternative electron acceptors in anaerobic respiration. These acceptors, such as nitrate (NO₃⁻), sulfate (SO₄²⁻), or carbon dioxide (CO₂), have lower electronegativity than oxygen, resulting in less energy being generated. While they provide a means for survival in oxygen-deprived environments, they are significantly less efficient than oxygen-based respiration.
Fermentation: A Quick Fix for Energy Production
Fermentation is a less efficient process that doesn't involve the ETC. It generates a small amount of ATP through substrate-level phosphorylation, a process where ATP is directly produced during glycolysis. Fermentation regenerates NAD⁺, allowing glycolysis to continue, even in the absence of oxygen. However, it yields far less ATP than aerobic respiration. Examples of fermentation include lactic acid fermentation (in muscle cells) and alcoholic fermentation (in yeast).
The Evolutionary Significance of Oxygen as the Final Electron Acceptor
The evolution of oxygenic photosynthesis, the process by which plants and cyanobacteria produce oxygen as a byproduct, dramatically changed the Earth's atmosphere and paved the way for the evolution of aerobic respiration. The abundance of oxygen allowed for the development of much more efficient energy-producing pathways, leading to the diversification and complexity of life forms.
Aerobic respiration, with oxygen as the final electron acceptor, is remarkably efficient at extracting energy from glucose. This efficiency fueled the evolution of larger, more complex organisms with higher energy demands.
Oxygen's Role Beyond Respiration: Reactive Oxygen Species and Antioxidants
While oxygen is crucial for life, its high reactivity can also lead to the formation of reactive oxygen species (ROS), such as superoxide radicals (O₂⁻) and hydrogen peroxide (H₂O₂). These ROS can damage cellular components, including DNA, proteins, and lipids. However, organisms have evolved various antioxidant defense mechanisms to mitigate ROS damage. These antioxidants neutralize ROS, protecting cells from oxidative stress.
Oxygen Deprivation: Hypoxia and Its Effects
Oxygen deprivation, or hypoxia, can have severe consequences for organisms. It leads to a reduction in ATP production, which can affect various cellular functions. Prolonged hypoxia can cause cell death and damage tissues and organs. The severity of hypoxia depends on the duration and extent of oxygen deprivation.
Conclusion: Oxygen – The Unsung Hero of Cellular Energy
The final electron acceptor in aerobic respiration, oxygen, is not merely a participant but the linchpin of the entire energy-generating process. Its high electronegativity allows for the efficient transfer of electrons, driving the production of ATP, the cell's primary energy currency. Understanding oxygen's pivotal role is essential for appreciating the complexities of cellular biology and the evolution of life on Earth. The consequences of oxygen deficiency highlight its crucial role in maintaining cellular function and overall organismal health. The interplay between oxygen, respiration, and antioxidant defense mechanisms reveals a fascinating and intricate biological system crucial for life as we know it. Further research continues to unravel the subtleties of this fundamental biological process and its implications for various aspects of human health and disease.
Latest Posts
Latest Posts
-
What Is The Oxidizing Agent In The Following Reaction
Mar 27, 2025
-
What Is The Most Abundant Fossil Fuel In The World
Mar 27, 2025
-
Which Of The Following Is Not Found In Rna
Mar 27, 2025
-
Choose The Correct Answer From The Given Options
Mar 27, 2025
-
In The Figure A Metal Rod Is Forced To Move
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about The Final Electron Acceptor In Aerobic Respiration Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
