What Is The Oxidizing Agent In The Following Reaction
News Leon
Mar 27, 2025 · 6 min read
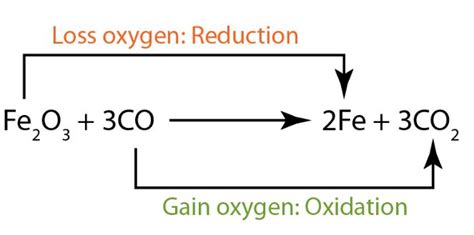
Table of Contents
Identifying the Oxidizing Agent: A Deep Dive into Redox Reactions
Understanding redox reactions is crucial in chemistry, and a key component of this understanding is identifying the oxidizing and reducing agents. This article will delve into the process of determining the oxidizing agent in a chemical reaction, providing a comprehensive guide with examples and explanations. We will explore the underlying principles of oxidation and reduction, discuss various methods for identifying oxidizing agents, and look at real-world applications of this knowledge.
What are Oxidation and Reduction?
Before we can identify the oxidizing agent, we need a solid understanding of oxidation and reduction themselves. These terms, often shortened to "redox," describe the transfer of electrons between chemical species.
-
Oxidation: Oxidation is the loss of electrons by a species. This results in an increase in the oxidation state (oxidation number) of the element involved. A species that undergoes oxidation is called a reducing agent because it causes another species to be reduced.
-
Reduction: Reduction is the gain of electrons by a species. This results in a decrease in the oxidation state of the element involved. A species that undergoes reduction is called an oxidizing agent because it causes another species to be oxidized.
The Key to Identifying Oxidizing Agents: Oxidation State Changes
The most reliable method for identifying the oxidizing agent involves tracking the changes in oxidation states of the elements involved in the reaction. Remember:
- The oxidizing agent is reduced itself. It gains electrons and its oxidation state decreases.
- The reducing agent is oxidized itself. It loses electrons and its oxidation state increases.
Step-by-Step Guide to Identifying the Oxidizing Agent
Let's break down the process with a structured approach:
-
Write the Balanced Chemical Equation: You need a correctly balanced equation to accurately assess the electron transfer. Make sure all atoms and charges are balanced on both sides of the equation.
-
Assign Oxidation States: Assign oxidation states to all elements in both the reactants and products. Remember the rules for assigning oxidation states, which include:
- The oxidation state of an element in its free (uncombined) state is always 0. (e.g., O₂ has an oxidation state of 0 for each oxygen atom).
- The oxidation state of a monatomic ion is equal to its charge. (e.g., Cl⁻ has an oxidation state of -1).
- The sum of oxidation states in a neutral compound is 0.
- The sum of oxidation states in a polyatomic ion is equal to the charge of the ion.
- In most compounds, the oxidation state of hydrogen is +1. The exception is metal hydrides, where it is -1.
- In most compounds, the oxidation state of oxygen is -2. Exceptions include peroxides (e.g., H₂O₂, where it's -1) and superoxides.
- Group 1 elements have an oxidation state of +1.
- Group 2 elements have an oxidation state of +2.
- Fluorine always has an oxidation state of -1.
-
Identify Changes in Oxidation States: Compare the oxidation states of each element in the reactants to its oxidation state in the products. Look for increases (oxidation) and decreases (reduction).
-
Identify the Oxidizing Agent: The species whose element shows a decrease in oxidation state is the oxidizing agent. This species has gained electrons and caused the oxidation of another species.
Illustrative Examples
Let's apply this process to some examples. Remember that you need to provide the specific reaction you want analyzed for a definitive answer. However, here are some general examples that showcase the methodology:
Example 1: Reaction of Zinc with Copper(II) Sulfate
The reaction is: Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
-
Balanced Equation: The equation is already balanced.
-
Oxidation States:
- Zn(s): Oxidation state of Zn = 0
- Cu in CuSO₄: Oxidation state of Cu = +2
- S in CuSO₄: Oxidation state of S = +6
- O in CuSO₄: Oxidation state of O = -2
- Zn in ZnSO₄: Oxidation state of Zn = +2
- Cu(s): Oxidation state of Cu = 0
- S in ZnSO₄: Oxidation state of S = +6
- O in ZnSO₄: Oxidation state of O = -2
-
Changes in Oxidation States:
- Zn goes from 0 to +2 (oxidation)
- Cu goes from +2 to 0 (reduction)
-
Oxidizing Agent: CuSO₄ is the oxidizing agent because Cu within it is reduced (its oxidation state decreases).
Example 2: Reaction of Potassium Permanganate with Hydrochloric Acid
This is a more complex example: 2KMnO₄ + 16HCl → 2KCl + 2MnCl₂ + 5Cl₂ + 8H₂O
-
Balanced Equation: The equation is already balanced.
-
Oxidation States: (This requires careful application of the rules)
- KMnO₄: K = +1, Mn = +7, O = -2
- HCl: H = +1, Cl = -1
- KCl: K = +1, Cl = -1
- MnCl₂: Mn = +2, Cl = -1
- Cl₂: Cl = 0
- H₂O: H = +1, O = -2
-
Changes in Oxidation States:
- Mn goes from +7 to +2 (reduction)
- Cl goes from -1 to 0 (oxidation)
-
Oxidizing Agent: KMnO₄ is the oxidizing agent because Mn within it is reduced. Note that some of the Cl is oxidized, making HCl the reducing agent.
Example 3: Combustion of Methane
CH₄ + 2O₂ → CO₂ + 2H₂O
-
Balanced Equation: The equation is already balanced.
-
Oxidation States:
- CH₄: C = -4, H = +1
- O₂: O = 0
- CO₂: C = +4, O = -2
- H₂O: H = +1, O = -2
-
Changes in Oxidation States:
- C goes from -4 to +4 (oxidation)
- O goes from 0 to -2 (reduction)
-
Oxidizing Agent: O₂ is the oxidizing agent because the oxygen atoms are reduced.
Beyond Simple Examples: Disproportionation Reactions
In disproportionation reactions, the same element undergoes both oxidation and reduction. Identifying the oxidizing agent becomes more subtle here. The species containing the element that is reduced is considered the oxidizing agent, even though part of that same species is oxidized.
Applications of Identifying Oxidizing Agents
The ability to identify oxidizing agents has numerous applications:
-
Corrosion Prevention: Understanding oxidizing agents is crucial in preventing corrosion of metals. Protecting metals from reacting with oxidizing agents in the environment is vital for maintaining their structural integrity.
-
Industrial Processes: Many industrial processes rely on redox reactions, such as the production of metals from their ores (metallurgy). Precise control over oxidizing and reducing agents is critical.
-
Organic Chemistry: In organic chemistry, many reactions involve oxidation and reduction, influencing the properties and reactivity of organic molecules. Oxidizing agents are frequently used to synthesize complex molecules.
-
Biology: Redox reactions are fundamental to biological processes such as cellular respiration, photosynthesis, and metabolism. Oxidizing agents play a key role in these energy-transfer processes.
Conclusion
Identifying the oxidizing agent in a redox reaction is a fundamental skill in chemistry. By carefully tracking oxidation state changes, you can pinpoint the species responsible for causing the oxidation of another species. This knowledge is essential for understanding countless chemical processes, from industrial applications to biological phenomena. Always remember to start with a balanced chemical equation and diligently apply the rules of oxidation state assignment. Mastering this process allows for deeper understanding of the chemical world around us.
Latest Posts
Latest Posts
-
Where Do Transcription And Translation Occur In Prokaryotic Cells
Mar 30, 2025
-
Groups Of Cells That Are Similar In Structure And Function
Mar 30, 2025
-
Indicate Whether The Following Statements Are True Or False
Mar 30, 2025
-
Which Of The Following Is Not A Conductor
Mar 30, 2025
-
The Dissociation Of A Weak Electrolyte Is Suppressed When
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidizing Agent In The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
