The Conjugate Acid Of H2o Is
News Leon
Mar 19, 2025 · 6 min read
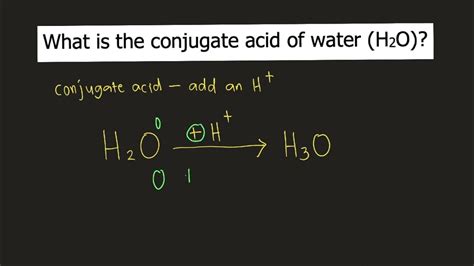
Table of Contents
The Conjugate Acid of H₂O Is: Understanding Acids, Bases, and Conjugate Pairs
Water (H₂O) is a ubiquitous molecule, crucial to life and playing a central role in countless chemical reactions. Understanding its behavior as an acid and a base, and specifically identifying its conjugate acid, is fundamental to grasping acid-base chemistry. This article delves deep into the concept of conjugate acid-base pairs, explains how water acts in different contexts, and definitively answers the question: the conjugate acid of H₂O is H₃O⁺, the hydronium ion.
Understanding Acids and Bases: A Quick Refresher
Before we delve into the specifics of water's conjugate acid, let's briefly review the fundamental definitions of acids and bases. Several theories exist, but the most relevant for this discussion is the Brønsted-Lowry theory.
According to the Brønsted-Lowry theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. This proton transfer is the key to acid-base reactions. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid.
This relationship is crucial because it highlights the dynamic nature of acid-base reactions. The acid and its conjugate base, and the base and its conjugate acid, form a conjugate pair. They are related by the difference of a single proton.
Water: Amphoteric Behavior
Water exhibits a unique property: it's amphoteric. This means it can act as both an acid and a base, depending on the reaction context.
Water as an Acid
In the presence of a strong base, like hydroxide ion (OH⁻), water can act as an acid, donating a proton:
H₂O + OH⁻ ⇌ OH⁻ + H₃O⁺
Here, water donates a proton (H⁺) to the hydroxide ion, forming the hydroxide ion (OH⁻) and the hydronium ion (H₃O⁺). Notice that the water molecule loses a proton, fulfilling the definition of an acid.
Water as a Base
Conversely, in the presence of a strong acid, like hydrochloric acid (HCl), water acts as a base, accepting a proton:
HCl + H₂O ⇌ Cl⁻ + H₃O⁺
In this case, water accepts a proton from HCl, forming the chloride ion (Cl⁻) and the hydronium ion (H₃O⁺). Here, water gains a proton, fitting the definition of a base.
Identifying the Conjugate Acid of H₂O
Now, let's directly address the central question: What is the conjugate acid of H₂O?
Based on the Brønsted-Lowry theory and the examples above, when water acts as a base and accepts a proton, it transforms into H₃O⁺, the hydronium ion. Therefore, the conjugate acid of H₂O is H₃O⁺.
This is the key takeaway: the conjugate acid is simply the species formed after the base accepts a proton. This proton addition is the defining characteristic of conjugate acid formation.
The Importance of Hydronium Ion (H₃O⁺)
The hydronium ion is not merely a theoretical entity; it plays a crucial role in aqueous solutions. It's the dominant form of protons (H⁺) in water. Free protons (H⁺) are extremely reactive and do not exist independently in aqueous solutions. Instead, they are always associated with a water molecule, forming the more stable hydronium ion.
The concentration of hydronium ions ([H₃O⁺]) directly determines the acidity or pH of a solution. The pH scale is a logarithmic scale that expresses the concentration of hydronium ions:
pH = -log₁₀[H₃O⁺]
A lower pH indicates a higher concentration of H₃O⁺ and thus a more acidic solution.
Conjugate Acid-Base Pairs in Action: More Examples
To further solidify the understanding of conjugate acid-base pairs, let's look at a few more examples:
-
Ammonia (NH₃) and Ammonium Ion (NH₄⁺): Ammonia acts as a base, accepting a proton to form its conjugate acid, the ammonium ion.
-
Acetic Acid (CH₃COOH) and Acetate Ion (CH₃COO⁻): Acetic acid acts as an acid, donating a proton to form its conjugate base, the acetate ion.
-
Carbonic Acid (H₂CO₃) and Bicarbonate Ion (HCO₃⁻): Carbonic acid acts as an acid, losing a proton to form its conjugate base, the bicarbonate ion. The bicarbonate ion can further act as an acid, losing another proton to form its conjugate base, the carbonate ion (CO₃²⁻). This demonstrates that a species can have multiple conjugate pairs depending on the reaction.
The Significance of Conjugate Acid-Base Pairs in Biological Systems
Conjugate acid-base pairs are not just a theoretical concept; they have immense significance in biological systems. Many biological molecules, including amino acids, proteins, and nucleic acids, contain functional groups that can act as acids or bases. These molecules often participate in acid-base reactions to maintain the delicate pH balance necessary for biological processes.
For instance, phosphate buffers in our blood utilize the conjugate acid-base pair of H₂PO₄⁻ and HPO₄²⁻ to resist pH changes. This buffering capacity is crucial for maintaining the stable internal environment required for proper cellular function.
Applications in Various Fields
The concept of conjugate acid-base pairs has broad applications beyond biology:
-
Environmental Science: Understanding acid-base chemistry is vital for assessing water quality and controlling pollution. Conjugate acid-base systems are involved in many environmental processes, like acid rain and soil chemistry.
-
Analytical Chemistry: Titrations, a crucial analytical technique, rely heavily on acid-base reactions and the concept of conjugate pairs to determine the concentration of unknown substances.
-
Industrial Chemistry: Many industrial processes, like the production of fertilizers and pharmaceuticals, involve acid-base reactions where the knowledge of conjugate acid-base pairs is essential for process optimization and control.
Beyond the Basics: Acid Dissociation Constant (Ka) and pKa
A crucial aspect of understanding acid-base chemistry is the concept of the acid dissociation constant (Ka) and its logarithmic equivalent, pKa. Ka is the equilibrium constant for the dissociation of an acid in water. A larger Ka value indicates a stronger acid, meaning it readily donates its proton. The pKa is simply -log₁₀Ka. A lower pKa signifies a stronger acid.
For water, the autoionization constant (Kw) is used, representing the equilibrium constant for the self-ionization of water:
2H₂O ⇌ H₃O⁺ + OH⁻
Kw = [H₃O⁺][OH⁻] = 1.0 x 10⁻¹⁴ at 25°C
This constant is fundamental in understanding the pH of pure water and aqueous solutions.
Conclusion: The Hydronium Ion as the Cornerstone of Aqueous Acid-Base Chemistry
In conclusion, the conjugate acid of H₂O is undeniably H₃O⁺, the hydronium ion. This seemingly simple answer forms the cornerstone of understanding acid-base chemistry in aqueous solutions. The amphoteric nature of water, its ability to act as both an acid and a base, highlights the dynamic equilibrium present in many chemical systems. Furthermore, the significance of conjugate acid-base pairs extends far beyond theoretical chemistry, finding applications across various scientific and industrial disciplines. From biological systems maintaining pH homeostasis to industrial processes and environmental monitoring, the understanding of conjugate acid-base pairs is indispensable. A thorough grasp of these concepts provides a solid foundation for further exploration into the fascinating world of acid-base chemistry.
Latest Posts
Latest Posts
-
36 Rounded To The Nearest Ten
Mar 19, 2025
-
Oxidation State Of Cr In Cr2o72
Mar 19, 2025
-
Which Of The Following Is A Scalar Quantity
Mar 19, 2025
-
Correctly Identify The Following Formed Elements
Mar 19, 2025
-
Points That Lie On The Same Line
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about The Conjugate Acid Of H2o Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
