Oxidation State Of Cr In Cr2o72
News Leon
Mar 19, 2025 · 5 min read
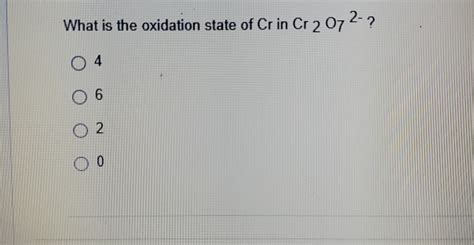
Table of Contents
Understanding the Oxidation State of Cr in Cr₂O₇²⁻ (Dichromate Ion)
The dichromate ion, Cr₂O₇²⁻, is a powerful oxidizing agent commonly used in various chemical applications, from laboratory experiments to industrial processes. Understanding the oxidation state of chromium (Cr) within this ion is crucial for predicting its reactivity and its role in redox reactions. This article will delve deep into determining and understanding the oxidation state of chromium in Cr₂O₇²⁻, exploring the underlying principles and providing a comprehensive overview.
Defining Oxidation State
Before we embark on determining the oxidation state of chromium in the dichromate ion, let's establish a clear understanding of the concept. The oxidation state, also known as the oxidation number, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool in understanding redox reactions, where electrons are transferred between atoms. While not a true charge, it's a valuable concept for predicting the behavior of elements in chemical reactions.
Determining the Oxidation State of Cr in Cr₂O₇²⁻
To determine the oxidation state of chromium in Cr₂O₇²⁻, we need to consider the following:
- The overall charge of the ion: The dichromate ion carries a 2- charge (Cr₂O₇²⁻).
- The oxidation state of oxygen: Oxygen, almost universally, has an oxidation state of -2 in its compounds, except in peroxides (where it's -1) and with fluorine (where it's positive).
- The principle of charge neutrality: The sum of the oxidation states of all atoms in the ion must equal the overall charge of the ion.
Let's apply these principles:
Let 'x' represent the oxidation state of chromium (Cr). Since there are two chromium atoms in the dichromate ion, the total contribution from chromium is 2x. Oxygen has an oxidation state of -2, and there are seven oxygen atoms, contributing a total of 7(-2) = -14.
Therefore, the equation for charge neutrality becomes:
2x + (-14) = -2
Solving for x:
2x = -2 + 14 2x = 12 x = +6
Therefore, the oxidation state of chromium (Cr) in Cr₂O₇²⁻ is +6.
Implications of the +6 Oxidation State
The +6 oxidation state of chromium in the dichromate ion has significant implications for its chemical behavior:
-
Strong Oxidizing Agent: Chromium in its +6 oxidation state is a powerful oxidizing agent. This means it readily accepts electrons from other species, undergoing reduction itself in the process. This high oxidizing power is attributed to the relatively high electronegativity of chromium and its strong tendency to achieve a more stable lower oxidation state.
-
Redox Reactions: Dichromate's strong oxidizing ability makes it a central player in many redox reactions. In these reactions, dichromate is reduced, typically to Cr³⁺ (chromium(III) ion), while another species is oxidized. The color change from orange (Cr₂O₇²⁻) to green (Cr³⁺) is often used to visually monitor these reactions. This color change is a key indicator of the redox reaction's completion.
-
Applications in Chemistry: The strong oxidizing properties of Cr₂O₇²⁻ are exploited in diverse applications:
- Organic Chemistry: It's employed as an oxidizing agent in various organic reactions, such as the oxidation of alcohols to ketones or aldehydes.
- Analytical Chemistry: It's utilized in titrations to determine the concentration of reducing agents. The sharp color change at the endpoint makes it a convenient reagent for such purposes.
- Industrial Processes: Dichromate finds applications in electroplating, leather tanning, and other industrial processes requiring a strong oxidizing agent. However, due to its toxicity and environmental concerns, its use is increasingly being replaced by greener alternatives.
Further Exploration: Structure and Bonding
Beyond the oxidation state, understanding the structure and bonding in the dichromate ion provides a deeper insight into its properties:
-
Chromate (CrO₄²⁻) vs. Dichromate (Cr₂O₇²⁻): The dichromate ion is formed from the condensation of two chromate ions (CrO₄²⁻). In acidic solutions, the equilibrium favors the dichromate formation. The equilibrium between chromate and dichromate is pH-dependent; chromate predominates in basic solutions, while dichromate is more prevalent in acidic solutions.
-
Molecular Structure: The dichromate ion has a linear structure where two tetrahedral CrO₄ units share one oxygen atom. This bridging oxygen atom accounts for the characteristic linear arrangement of the two tetrahedra. This structure plays a significant role in the ion's reactivity and stability.
-
Bonding: The bonding in the dichromate ion is complex and can be described using various models, including valence bond theory and molecular orbital theory. Both covalent and ionic character contribute to the overall bonding in the structure. A detailed description of this bonding is beyond the scope of this article, but the understanding of its complexity is essential to appreciating the ion's properties.
Safety Precautions and Environmental Concerns
It's crucial to acknowledge the safety precautions associated with handling dichromate and its related compounds:
-
Toxicity: Chromium(VI) compounds, including dichromate, are highly toxic and carcinogenic. Appropriate safety measures, such as wearing protective gloves, eye protection, and lab coats, are necessary when handling these substances.
-
Environmental Impact: The release of chromium(VI) compounds into the environment is detrimental to ecosystems and human health. Its toxicity and persistence in the environment necessitate the careful management and disposal of dichromate waste. The use of greener and more sustainable alternatives is becoming increasingly important to mitigate the environmental impact of chromium(VI) compounds.
Conclusion
The oxidation state of chromium in Cr₂O₇²⁻ is +6, a key factor in its strong oxidizing properties and diverse applications. Understanding this oxidation state, along with its structural features and bonding characteristics, is fundamental to comprehending its role in various chemical processes. While its powerful oxidizing properties offer advantages in several applications, the toxicity and environmental concerns associated with chromium(VI) necessitate careful handling and a constant search for environmentally friendly alternatives. Further research and development of sustainable alternatives to dichromate are crucial to balance its usefulness with environmental protection and human health. This article offers a comprehensive overview of this important chemical species, highlighting its significance and potential hazards.
Latest Posts
Latest Posts
-
How Many H Bonds Between A And T
Mar 19, 2025
-
What Is The Largest Gland In The Body
Mar 19, 2025
-
Which Of The Following Is Rational
Mar 19, 2025
-
The Word Atom Comes From A Greek Word That Means
Mar 19, 2025
-
Moment Of Inertia For Hollow Sphere
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Oxidation State Of Cr In Cr2o72 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
