That Which Occupies Space And Has Mass
News Leon
Mar 21, 2025 · 6 min read
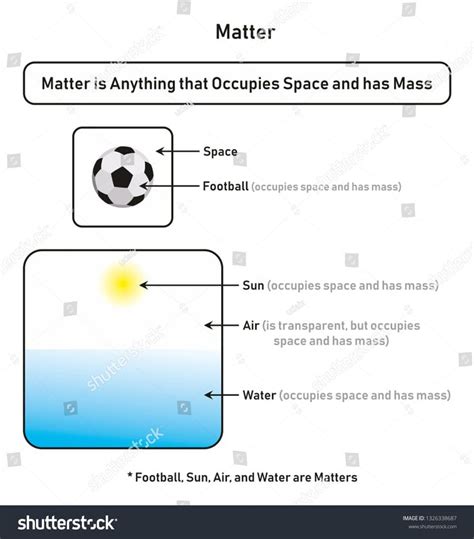
Table of Contents
That Which Occupies Space and Has Mass: Exploring Matter in the Universe
Matter. A seemingly simple concept, yet one that underpins the entire universe. From the smallest subatomic particle to the largest galaxy, everything we see, touch, and interact with is composed of matter. But what exactly is matter? This seemingly straightforward question opens the door to a fascinating exploration of physics, chemistry, and the fundamental building blocks of reality. This article will delve deep into the nature of matter, exploring its properties, classifications, states, and its crucial role in shaping the cosmos.
Defining Matter: A Foundation of Physics
At its core, matter is defined as anything that occupies space and has mass. This seemingly simple definition encapsulates a profound truth: matter isn't just something that exists; it exists somewhere and possesses an intrinsic property called mass, which is a measure of its inertia – its resistance to changes in motion. The more massive an object, the harder it is to accelerate or decelerate.
This definition, however, is a starting point. To truly understand matter, we need to dissect its properties and explore its various forms.
Properties of Matter: Distinguishing Characteristics
Matter exhibits a range of properties that allow us to distinguish one substance from another. These properties can be broadly categorized as:
1. Physical Properties:
These are characteristics that can be observed or measured without changing the chemical composition of the matter. Examples include:
- Mass: A fundamental property representing the amount of matter in an object.
- Volume: The amount of three-dimensional space occupied by matter.
- Density: The mass per unit volume of a substance.
- Melting point: The temperature at which a solid transforms into a liquid.
- Boiling point: The temperature at which a liquid transforms into a gas.
- Color: The visual perception of light reflected or emitted by a substance.
- Texture: The surface feel of a substance (smooth, rough, etc.).
- Conductivity: The ability of a substance to conduct heat or electricity.
- Malleability: The ability of a substance to be hammered into thin sheets.
- Ductility: The ability of a substance to be drawn into wires.
2. Chemical Properties:
These properties describe how a substance behaves when it interacts with other substances, undergoing a change in its chemical composition. Examples include:
- Reactivity: The tendency of a substance to undergo chemical reactions.
- Flammability: The ability of a substance to burn in the presence of oxygen.
- Toxicity: The degree to which a substance is harmful to living organisms.
- Acidity/Alkalinity (pH): A measure of the concentration of hydrogen ions in a solution.
Understanding both physical and chemical properties is crucial for identifying, classifying, and utilizing various forms of matter.
States of Matter: From Solid to Plasma
Matter exists in various states, determined primarily by the arrangement and energy of its constituent particles. The most common states are:
1. Solid:
In a solid state, particles are tightly packed together in a fixed arrangement, resulting in a definite shape and volume. Solids generally have high density and are relatively incompressible. Examples include ice, rocks, and metals.
2. Liquid:
Liquids have particles that are closer together than gases but not as tightly packed as solids. They have a definite volume but take the shape of their container. Liquids are relatively incompressible and have a lower density than solids. Examples include water, oil, and mercury.
3. Gas:
Gases have particles that are widely dispersed and move freely. They have neither a definite shape nor a definite volume, expanding to fill the available space. Gases are easily compressible and have low density. Examples include air, oxygen, and carbon dioxide.
4. Plasma:
Plasma is often considered the fourth state of matter. It's an electrically charged gas composed of ions and free electrons. Plasma occurs at extremely high temperatures, where electrons are stripped from atoms. Examples include lightning, the sun, and fluorescent lights.
5. Bose-Einstein Condensate (BEC):
This exotic state of matter occurs at extremely low temperatures, near absolute zero. Atoms in a BEC lose their individual identities and behave as a single quantum entity. It exhibits fascinating properties, including superfluidity (flowing without resistance).
Classification of Matter: Elements, Compounds, and Mixtures
Matter can also be classified based on its chemical composition:
1. Elements:
Elements are pure substances that cannot be broken down into simpler substances by chemical means. Each element is composed of atoms with the same number of protons in their nucleus. The periodic table organizes all known elements based on their atomic number and properties. Examples include hydrogen (H), oxygen (O), and gold (Au).
2. Compounds:
Compounds are substances formed when two or more elements combine chemically in a fixed ratio. The properties of a compound are different from the properties of its constituent elements. Compounds can be broken down into simpler substances through chemical reactions. Examples include water (H₂O), table salt (NaCl), and carbon dioxide (CO₂).
3. Mixtures:
Mixtures are combinations of two or more substances that are not chemically bonded. Mixtures can be homogeneous (uniform composition throughout, like saltwater) or heterogeneous (non-uniform composition, like sand and water). The components of a mixture can be separated by physical means.
The Role of Matter in the Universe
Matter plays a fundamental role in shaping the universe. Stars are massive spheres of plasma, undergoing nuclear fusion to produce energy and heavier elements. Planets are formed from the accretion of matter in the early solar system. Galaxies are vast collections of stars, gas, and dust, bound together by gravity. The entire universe, as we know it, is made of matter. Even the seemingly empty space between celestial bodies contains a sparse distribution of matter, including dark matter, a mysterious substance that interacts gravitationally but doesn't emit or absorb light.
Exploring Further: Beyond the Basics
The study of matter is a vast and ongoing field of research. Modern physics delves into the subatomic world, exploring the properties of quarks, leptons, and bosons – the fundamental particles that constitute all matter. Quantum mechanics provides a framework for understanding the behavior of matter at the atomic and subatomic levels, revealing the probabilistic nature of particles and their interactions.
The study of materials science focuses on the properties and applications of various materials, ranging from metals and ceramics to polymers and composites. Nanotechnology explores the manipulation of matter at the nanoscale, leading to innovations in medicine, electronics, and energy production. Cosmology investigates the origin, evolution, and large-scale structure of the universe, exploring the role of dark matter and dark energy in the cosmic expansion.
Understanding matter is crucial for numerous scientific and technological advancements. Developing new materials with specific properties, designing efficient energy systems, and exploring the possibilities of space travel all rely on a deep understanding of the fundamental nature of matter. From the smallest particle to the largest galaxy, the study of matter continues to unravel the mysteries of our universe and drive human innovation. The journey of discovery is far from over, with countless questions remaining to be answered about this fundamental building block of our reality. As we continue to probe deeper into the secrets of matter, we gain a richer understanding of the universe and our place within it.
Latest Posts
Latest Posts
-
How Many Bonds Can Beryllium Form
Mar 22, 2025
-
Which Of The Following Graphs Represents A Function
Mar 22, 2025
-
A Ball Of Hot Glowing Gases
Mar 22, 2025
-
What Is The Measure Of Ced
Mar 22, 2025
-
Water Is To A Pipe As Is To A Wire
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about That Which Occupies Space And Has Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
