Temperature Measure Of Average Molecular Translational Kinestic Energty
News Leon
Mar 16, 2025 · 6 min read
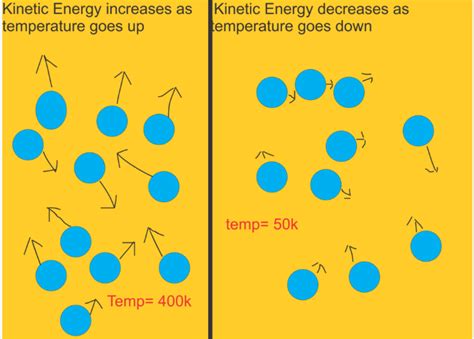
Table of Contents
Temperature: A Measure of Average Molecular Translational Kinetic Energy
Temperature, a fundamental concept in physics and everyday life, is more than just a measure of how hot or cold something feels. At its core, temperature is a direct reflection of the average translational kinetic energy of the particles (atoms or molecules) within a substance. This relationship provides a powerful link between the macroscopic world we experience and the microscopic world of atoms and molecules. Understanding this connection unlocks a deeper appreciation of thermodynamics, statistical mechanics, and various applications across diverse scientific fields.
The Microscopic Dance: Translational Kinetic Energy
Before diving into the relationship between temperature and kinetic energy, let's define the key players. Kinetic energy is the energy an object possesses due to its motion. For a molecule, this motion can manifest in several ways:
- Translational kinetic energy: This refers to the energy associated with the molecule moving from one place to another. It's the linear motion of the molecule's center of mass. Think of a ball rolling across a table – its kinetic energy is translational.
- Rotational kinetic energy: This is the energy associated with the molecule rotating around its center of mass. Imagine a spinning top; its kinetic energy is largely rotational.
- Vibrational kinetic energy: This type of energy arises from the vibrational motion of atoms within a molecule. Think of the atoms within a molecule oscillating back and forth like springs.
While all these forms of kinetic energy contribute to the overall energy of a molecule, temperature is specifically a measure of the average translational kinetic energy of the molecules. This is because translational motion is directly related to how a substance interacts with its surroundings and transfers heat.
The Importance of "Average"
It's crucial to emphasize the word "average." In any macroscopic sample of a substance, the molecules are not all moving at the same speed. They have a distribution of speeds, with some moving incredibly fast, some moving slowly, and most somewhere in between. Temperature represents the average kinetic energy of this distribution. This average kinetic energy is directly proportional to the absolute temperature.
Connecting Temperature and Kinetic Energy: The Boltzmann Constant
The relationship between the average translational kinetic energy (KE<sub>avg</sub>) and the absolute temperature (T) is elegantly described by the following equation:
KE<sub>avg</sub> = (3/2)kT
Where:
- KE<sub>avg</sub> is the average translational kinetic energy of a molecule.
- k is the Boltzmann constant (approximately 1.38 × 10<sup>-23</sup> J/K).
- T is the absolute temperature in Kelvin (K).
This equation reveals the fundamental connection: as temperature increases, the average translational kinetic energy of the molecules increases proportionally. This is why hotter objects feel hotter – their molecules are moving faster on average. Conversely, cooler objects have molecules moving slower on average.
Understanding the Kelvin Scale
The use of the Kelvin scale (K) in this equation is not arbitrary. The Kelvin scale is an absolute temperature scale, meaning its zero point (0 K) corresponds to absolute zero, the theoretical temperature at which all molecular motion ceases. This contrasts with the Celsius and Fahrenheit scales, which have arbitrary zero points. The absolute temperature scale ensures a direct and linear relationship between temperature and average kinetic energy.
Implications and Applications
The relationship between temperature and average molecular translational kinetic energy has profound implications across numerous scientific and engineering disciplines:
1. Thermodynamics:
Understanding this relationship is fundamental to thermodynamics, which deals with heat and work. The transfer of heat between objects is essentially the transfer of kinetic energy from molecules in one object to molecules in another. The direction of heat flow is always from a region of higher average kinetic energy (higher temperature) to a region of lower average kinetic energy (lower temperature).
2. Statistical Mechanics:
Statistical mechanics utilizes this connection to connect the macroscopic properties of matter (like temperature and pressure) to the microscopic behavior of its constituent particles. By considering the statistical distribution of molecular speeds and energies, statistical mechanics can predict macroscopic properties based on microscopic interactions.
3. Gas Laws:
The ideal gas law, a cornerstone of physical chemistry, is directly linked to the average kinetic energy of gas molecules. The pressure exerted by a gas is a result of the collisions of gas molecules with the walls of the container. The frequency and force of these collisions are directly related to the average kinetic energy of the molecules, which is, in turn, determined by the temperature.
4. Material Science:
Temperature influences the properties of materials in countless ways. The thermal expansion of materials, for instance, is a direct consequence of the increased average molecular kinetic energy at higher temperatures leading to greater intermolecular distances. Similarly, the phase transitions (solid, liquid, gas) of a substance are governed by the balance between the average kinetic energy of the molecules and the intermolecular forces holding them together.
5. Chemistry:
Chemical reaction rates are highly temperature-dependent. Higher temperatures lead to a greater proportion of molecules having sufficient kinetic energy to overcome the activation energy barrier for a reaction to occur, thus increasing the reaction rate. This is a cornerstone principle of chemical kinetics.
6. Meteorology:
Temperature plays a critical role in meteorological processes. Temperature gradients in the atmosphere drive weather patterns, including wind, precipitation, and storm formation. The kinetic energy of air molecules is directly responsible for atmospheric pressure and the movement of air masses.
Beyond the Ideal: Real-World Considerations
While the equation KE<sub>avg</sub> = (3/2)kT provides a fundamental understanding of the relationship between temperature and kinetic energy, it holds true only for ideal gases. In real-world systems, several factors complicate the picture:
- Intermolecular forces: In liquids and solids, intermolecular forces (like van der Waals forces and hydrogen bonds) significantly influence the movement of molecules. These forces affect the average kinetic energy and the relationship between temperature and kinetic energy is not as straightforward as in an ideal gas.
- Non-ideal gases: At high pressures and low temperatures, real gases deviate from ideal gas behavior. Intermolecular forces and the finite size of gas molecules become significant, altering the relationship between temperature and kinetic energy.
- Quantum effects: At very low temperatures, quantum mechanical effects become important and the classical description of kinetic energy breaks down. The behavior of molecules at these temperatures requires a quantum mechanical treatment.
Conclusion: A Deeper Understanding of Temperature
Temperature, seemingly a simple concept, reveals its complexity and richness when understood at the molecular level. Its direct connection to the average translational kinetic energy of molecules underpins a vast array of phenomena in physics, chemistry, and engineering. Understanding this fundamental relationship is essential for grasping the workings of the universe around us, from the smallest molecules to the largest weather systems. By exploring the intricacies of this relationship, we gain a more profound appreciation of the power and elegance of the laws governing the physical world. Further research into the intricacies of these interactions continues to unveil new insights and applications, furthering our understanding of matter and energy at their most fundamental levels.
Latest Posts
Latest Posts
-
Is Cl An Acid Or Base
Mar 17, 2025
-
A Charge Of Uniform Linear Density
Mar 17, 2025
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Temperature Measure Of Average Molecular Translational Kinestic Energty . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
