Smallest Part Of An Element Or Compound
News Leon
Apr 01, 2025 · 6 min read
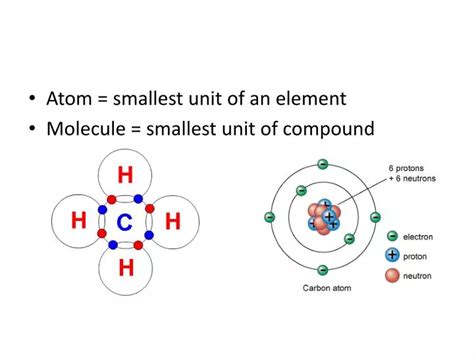
Table of Contents
Delving into the Atom: The Smallest Part of an Element or Compound
The quest to understand the fundamental building blocks of matter has captivated humankind for millennia. From ancient Greek philosophers pondering the nature of reality to modern-day physicists unraveling the mysteries of the quantum realm, the search for the smallest constituent of elements and compounds continues to drive scientific innovation. This journey leads us to the atom, the incredibly tiny particle that forms the basis of all chemical substances. But is the atom truly the smallest part? The answer, as we will explore, is nuanced and fascinating.
Atoms: The Fundamental Building Blocks
An atom is the smallest unit of matter that retains the chemical properties of an element. Each element, like hydrogen, oxygen, or gold, is defined by the number of protons in its atomic nucleus. These protons, along with neutrons (which are electrically neutral), reside in the atom's dense central core. Whizzing around this nucleus are electrons, negatively charged particles that are significantly lighter than protons and neutrons.
The arrangement of these subatomic particles dictates an atom's properties. The number of protons, also known as the atomic number, uniquely identifies the element. The total number of protons and neutrons is the atomic mass number. Isotopes are atoms of the same element with the same atomic number but different atomic mass numbers due to varying neutron counts.
Understanding Atomic Structure: A Deeper Dive
The structure of an atom is often simplified using models, such as the Bohr model, which depicts electrons orbiting the nucleus in specific energy levels or shells. While this model is helpful for visualizing atomic structure, it's crucial to understand its limitations. Modern quantum mechanics provides a more accurate, albeit more complex, description of the atom, using probability clouds rather than fixed orbits to represent the location of electrons. These clouds, also called orbitals, represent regions of space where there's a high probability of finding an electron.
The electrons in the outermost shell, known as valence electrons, play a crucial role in chemical bonding. These electrons are involved in interactions with other atoms, leading to the formation of molecules and compounds. The number of valence electrons largely determines an element's reactivity and its position in the periodic table.
Molecules and Compounds: Aggregates of Atoms
Atoms rarely exist in isolation. They tend to interact with each other, forming larger structures. A molecule is a group of two or more atoms held together by chemical bonds. These bonds arise from the electrostatic attraction between atoms, primarily involving their valence electrons.
A compound is a substance formed when two or more different elements are chemically bonded together. Water (H₂O), for instance, is a compound composed of two hydrogen atoms and one oxygen atom covalently bonded together. The properties of a compound are usually very different from the properties of its constituent elements. This demonstrates the transformative power of chemical bonding.
Types of Chemical Bonds: Covalent, Ionic, and Metallic
Several types of chemical bonds contribute to the formation of molecules and compounds. Covalent bonds involve the sharing of electrons between atoms, creating a strong attraction that holds them together. This type of bonding is prevalent in many organic molecules and compounds. Ionic bonds, on the other hand, involve the transfer of electrons from one atom to another, resulting in the formation of positively and negatively charged ions that are electrostatically attracted to each other. Table salt (NaCl) is a classic example of a compound formed through ionic bonding. Finally, metallic bonds are found in metals, where electrons are delocalized and shared among a lattice of metal atoms, contributing to their characteristic properties like conductivity and malleability.
Beyond the Atom: Subatomic Particles and the Quantum Realm
While the atom is considered the smallest unit of matter retaining the chemical properties of an element, it is far from indivisible. Delving deeper into the atom reveals a subatomic world governed by the laws of quantum mechanics. Protons and neutrons themselves are not fundamental particles but are composed of smaller constituents called quarks. These quarks are held together by the strong nuclear force, mediated by gluons. Electrons, on the other hand, are considered fundamental particles belonging to a class of particles called leptons.
The Standard Model of particle physics provides a comprehensive framework for understanding the fundamental particles and forces governing their interactions. This model includes not only quarks and leptons but also gauge bosons, which mediate the fundamental forces of nature, such as electromagnetism, the weak nuclear force, and the strong nuclear force. The Higgs boson, discovered in 2012, plays a crucial role in giving mass to particles.
The Uncertainty Principle and Quantum Superposition
The quantum realm is characterized by its inherent uncertainty. The Heisenberg uncertainty principle states that it's impossible to simultaneously know both the position and momentum of a particle with perfect accuracy. This principle highlights the probabilistic nature of quantum mechanics.
Another fascinating quantum phenomenon is superposition, where a particle can exist in multiple states simultaneously until measured. This implies that electrons, for instance, don't occupy a specific location until they are observed. These concepts are counterintuitive from a classical perspective but are essential for understanding the behavior of matter at the atomic and subatomic levels.
The Search for the "Smallest": A Continuous Journey
So, what is the smallest part of an element or compound? While the atom represents the smallest unit retaining the element's chemical properties, the subatomic world reveals a far richer and more complex reality. Quarks and leptons are currently considered fundamental particles, meaning they are not composed of smaller constituents (as far as we currently know). However, the search for even more fundamental particles continues, driven by ongoing research in particle physics.
The discovery of new particles and phenomena constantly challenges our understanding of the universe. String theory, for example, proposes that fundamental particles are not point-like objects but rather tiny vibrating strings, a concept that could potentially unify all fundamental forces and particles. Loop quantum gravity offers another alternative approach to unifying general relativity and quantum mechanics. These ongoing research endeavors push the boundaries of our knowledge and reveal the universe's intricate and fascinating architecture.
Conclusion: A Holistic Understanding
The quest to find the smallest part of an element or compound is a testament to humanity's relentless curiosity and intellectual drive. While the atom provides a foundational understanding of chemical properties, the exploration of the subatomic realm reveals layers of complexity governed by the principles of quantum mechanics. The ongoing search for fundamental particles and the development of unifying theories constantly reshape our understanding of the universe and its fundamental building blocks. Ultimately, appreciating the multifaceted nature of matter—from the atom's structure and bonding to the subatomic particles and forces that govern it—provides a more holistic and enriching perspective on the world around us. The journey of discovery is far from over; the pursuit of a complete understanding of the universe's smallest constituents continues to inspire and challenge scientists and researchers across the globe. The seemingly simple question—"What is the smallest part?"—leads us to a profoundly complex and captivating exploration of the fundamental nature of reality itself.
Latest Posts
Latest Posts
-
Are Metals Solid At Room Temperature
Apr 02, 2025
-
Which Of The Following Statements Correctly Describes Gene Linkage
Apr 02, 2025
-
Complete The Complementary Strand Of Dna
Apr 02, 2025
-
Is Carbon Dioxide A Pure Substance Or Mixture
Apr 02, 2025
-
The Given Reaction Proceeds In Two Parts
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Smallest Part Of An Element Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
