Saturation Water Vapor Pressure Increases With ___________ Temperature.
News Leon
Mar 29, 2025 · 6 min read
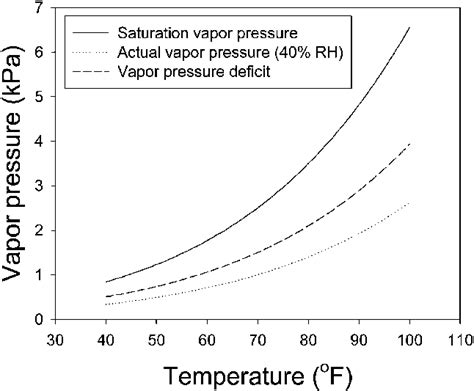
Table of Contents
Saturation Water Vapor Pressure Increases with Increasing Temperature
The relationship between saturation water vapor pressure (SWVP) and temperature is fundamental to understanding atmospheric processes, weather patterns, and numerous applications in various scientific and engineering fields. Simply put, saturation water vapor pressure increases with increasing temperature. This seemingly simple statement holds profound implications for everything from cloud formation to the design of industrial cooling systems. This comprehensive article will delve deep into this relationship, exploring its underlying physics, practical applications, and the subtle nuances that influence its behavior.
Understanding Saturation Water Vapor Pressure
Before exploring the temperature dependence, let's define what saturation water vapor pressure actually means. Imagine a closed container filled with water and air. Water molecules are constantly evaporating from the liquid surface and entering the gaseous phase as water vapor. Simultaneously, water vapor molecules in the air collide with the liquid surface and condense back into liquid water. When the rate of evaporation equals the rate of condensation, the air is said to be saturated with water vapor.
Saturation water vapor pressure (SWVP) is the partial pressure exerted by the water vapor in this saturated air. It represents the maximum amount of water vapor the air can hold at a given temperature and pressure. Crucially, this pressure is solely dependent on temperature. The higher the temperature, the greater the kinetic energy of water molecules, leading to a higher rate of evaporation and, consequently, a higher saturation vapor pressure.
The Physics Behind the Relationship
The increase in SWVP with temperature is governed by the Clausius-Clapeyron equation. This equation describes the relationship between the temperature and pressure at which two phases of a substance (in this case, liquid water and water vapor) are in equilibrium. While the full derivation is complex, the key takeaway is that the equation demonstrates an exponential relationship between temperature and SWVP. This means that a small increase in temperature leads to a significant increase in SWVP.
Molecular Dynamics and Evaporation
At the microscopic level, the relationship stems from the behavior of water molecules. Higher temperatures mean that more water molecules possess sufficient kinetic energy to overcome the intermolecular forces holding them in the liquid phase. This increased kinetic energy translates to a higher rate of evaporation, leading to a higher concentration of water vapor molecules in the air and, therefore, a higher SWVP.
Implications for Relative Humidity
Understanding the SWVP-temperature relationship is critical to interpreting relative humidity. Relative humidity is the ratio of the actual water vapor pressure in the air to the SWVP at the same temperature, expressed as a percentage. For instance, if the actual water vapor pressure is 10 hPa and the SWVP at a given temperature is 20 hPa, the relative humidity is 50%.
A key point to remember is that while the actual amount of water vapor in the air might remain constant, the relative humidity will change as the temperature changes because the SWVP changes. Warming the air decreases the relative humidity, even without a change in the amount of water vapor present. Conversely, cooling the air increases the relative humidity. This is why dew formation often occurs on cool surfaces at night – the air becomes saturated (100% relative humidity) as it cools, and excess water vapor condenses.
Practical Applications and Real-World Examples
The dependence of SWVP on temperature has far-reaching consequences in many fields:
Meteorology and Climatology
-
Cloud Formation: Clouds form when air containing water vapor cools and reaches saturation. As the air cools, the SWVP decreases, causing the relative humidity to increase. When the relative humidity reaches 100%, the air is saturated, and water vapor condenses into cloud droplets or ice crystals around condensation nuclei. This process is heavily influenced by the temperature-dependent nature of SWVP. Higher temperatures require more cooling to reach saturation, impacting cloud formation processes and precipitation patterns.
-
Precipitation: The amount and type of precipitation are directly linked to the SWVP. Warmer air can hold more water vapor, leading to potentially heavier rainfall events. However, the distribution of this precipitation is complex and also depends on other factors such as atmospheric stability and uplift mechanisms.
-
Humidity and Comfort: Human comfort is significantly impacted by relative humidity, which is intrinsically linked to SWVP and temperature. High temperatures combined with high humidity (meaning the actual vapor pressure is close to SWVP) create uncomfortable and potentially dangerous conditions due to reduced evaporative cooling of sweat. Conversely, low humidity, even at high temperatures, can feel more tolerable.
Engineering and Industrial Applications
-
Cooling Systems: Many cooling systems, such as air conditioners and refrigeration units, rely on the principle of latent heat of vaporization. The ability of a refrigerant to absorb heat as it evaporates and condense it as it returns to liquid form is directly connected to the SWVP. Understanding the SWVP-temperature relationship is crucial for optimizing the design and performance of these systems.
-
Drying Processes: In industrial drying processes, controlling the humidity of the surrounding air is critical. Knowledge of SWVP allows engineers to adjust temperature and airflow to achieve the desired drying rate.
-
HVAC Systems: Heating, ventilation, and air conditioning (HVAC) systems must account for the effect of temperature on SWVP to maintain optimal indoor comfort levels. Accurate modeling of SWVP is essential for efficient and effective climate control.
Agriculture and Horticulture
-
Plant Transpiration: Plants lose water through transpiration, a process influenced by the difference between the vapor pressure within the plant leaves and the SWVP of the surrounding air. Higher SWVP reduces the transpiration rate, potentially leading to water stress in plants, especially in hot and humid conditions.
-
Irrigation Management: Understanding SWVP helps optimize irrigation schedules. High SWVP indicates a higher potential for evaporation, necessitating more frequent watering to compensate for water loss.
Factors Affecting Saturation Water Vapor Pressure Beyond Temperature
While temperature is the primary factor influencing SWVP, other factors play a minor role:
-
Pressure: Although the effect is relatively small compared to temperature, an increase in atmospheric pressure slightly increases the SWVP. This is because higher pressure forces more water molecules into the gaseous phase.
-
Presence of other Gases: The presence of other gases in the air can have a minor impact on SWVP, particularly at high concentrations. This is primarily due to the intermolecular interactions between water vapor molecules and other gases.
These secondary factors are often negligible in most practical applications, and the temperature dependence remains the dominant influence on SWVP.
Conclusion
The relationship between saturation water vapor pressure and temperature is a fundamental concept with far-reaching consequences across numerous scientific and engineering disciplines. The exponential increase of SWVP with temperature is dictated by the Clausius-Clapeyron equation and explained by the increased kinetic energy of water molecules at higher temperatures. This relationship underpins crucial meteorological phenomena like cloud formation and precipitation, plays a vital role in the design of cooling and drying systems, and influences agricultural practices. Understanding this relationship provides valuable insights for mitigating the effects of climate change, improving technological efficiency, and enhancing human comfort and well-being. The accurate prediction and modeling of SWVP are essential for many applications and continue to be a focus of ongoing research and development.
Latest Posts
Latest Posts
-
What Is The Most Abundant Wbc
Apr 01, 2025
-
Domain And Range For Y 1 X
Apr 01, 2025
-
What Is The Mass Of A Beta Particle
Apr 01, 2025
-
12 Of 150 Is What Number
Apr 01, 2025
-
Draw The Organic Products Formed In The Following Reaction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Saturation Water Vapor Pressure Increases With ___________ Temperature. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
