What Is The Mass Of A Beta Particle
News Leon
Apr 01, 2025 · 6 min read
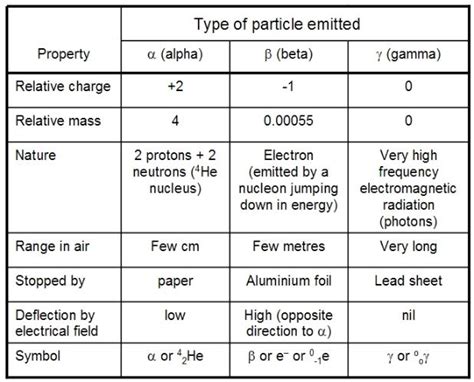
Table of Contents
What is the Mass of a Beta Particle? Unraveling the Mysteries of Beta Decay
Beta decay, a fundamental process in nuclear physics, plays a crucial role in the stability of atomic nuclei and the generation of energy in stars. Understanding this process requires grasping the properties of the beta particle itself, particularly its mass. This article delves into the intricacies of beta decay, focusing specifically on the mass of the beta particle and its implications for our understanding of nuclear physics.
Understanding Beta Decay: A Journey into the Nucleus
Beta decay is a type of radioactive decay in which a beta particle (β) is emitted from an atomic nucleus. There are two main types: beta-minus (β⁻) decay and beta-plus (β⁺) decay.
Beta-Minus (β⁻) Decay: The Electron's Role
In β⁻ decay, a neutron within the nucleus transforms into a proton, emitting an electron (the beta particle) and an antineutrino. This process can be represented by the following equation:
n → p + e⁻ + ν̅ₑ
Where:
- n represents a neutron
- p represents a proton
- e⁻ represents an electron (the beta particle)
- ν̅ₑ represents an electron antineutrino
The emission of the beta particle increases the atomic number of the nucleus by one while the mass number remains unchanged. This essentially transforms one element into another.
Beta-Plus (β⁺) Decay: Positron Emission
In β⁺ decay, a proton within the nucleus transforms into a neutron, emitting a positron (the beta particle) and a neutrino. The equation for this process is:
p → n + e⁺ + νₑ
Where:
- p represents a proton
- n represents a neutron
- e⁺ represents a positron (the beta particle)
- νₑ represents an electron neutrino
The emission of the positron decreases the atomic number of the nucleus by one, while again, the mass number remains unchanged. The positron, being the antiparticle of the electron, is also a beta particle.
The Mass of the Beta Particle: A Tale of Two Particles
The mass of a beta particle depends on whether it's an electron (β⁻) or a positron (β⁺). Since a positron is the antimatter counterpart of an electron, they have identical mass, but opposite charge.
Electron Mass: A Fundamental Constant
The rest mass of an electron, and therefore the mass of a beta-minus particle, is approximately 9.109 × 10⁻³¹ kilograms (kg) or 5.486 × 10⁻⁴ atomic mass units (amu). This value is a fundamental constant in physics and has been precisely measured. It's significantly smaller than the mass of a proton or neutron, which are the building blocks of the nucleus.
Positron Mass: Mirror Image of the Electron
As mentioned earlier, the positron, or beta-plus particle, possesses the same rest mass as the electron, approximately 9.109 × 10⁻³¹ kg or 5.486 × 10⁻⁴ amu. The only difference lies in its positive charge, contrasting with the electron's negative charge. This mass equality is a key prediction of the theory of special relativity and has been experimentally verified.
Relativistic Effects and Beta Particle Energy
While the rest mass of a beta particle is relatively small, its energy can be significant due to relativistic effects. Beta particles emitted during decay possess a range of energies, a continuous spectrum rather than a discrete set of values. This energy distribution is a consequence of the simultaneous emission of the beta particle and the neutrino (or antineutrino). The energy is shared between these two particles, resulting in a spectrum of beta particle energies.
The energy of a beta particle is related to its mass through Einstein's famous equation, E=mc². The higher the energy of the beta particle, the higher its relativistic mass. However, when discussing the "mass" of a beta particle, we usually refer to its rest mass, which is the mass when it is at rest. The relativistic mass increases with velocity, but the rest mass remains constant.
Implications of Beta Particle Mass in Nuclear Physics and Beyond
The small mass of the beta particle has significant implications for various aspects of physics:
-
Nuclear Stability: The mass difference between the parent and daughter nuclei in beta decay is related to the energy released in the process. The relatively small mass of the beta particle allows for the energy release to be distributed between the beta particle and the neutrino, contributing to the stability of some nuclei.
-
Nuclear Medicine: Beta decay is used in various medical applications, including cancer therapy. Beta emitters are used in radiotherapy because their beta particles can penetrate tissues and deliver radiation dose to cancerous cells, while minimizing damage to surrounding healthy tissues. The specific energy of the beta particles is important for targeting the tumor effectively.
-
Astrophysics: Beta decay plays a significant role in nucleosynthesis, the process by which elements are formed in stars. Understanding the mass and energy of beta particles is crucial for modeling stellar evolution and element formation.
-
Particle Physics: The beta decay process has provided crucial insights into the fundamental interactions between particles, namely the weak nuclear force. The discovery of the neutrino, alongside the beta particle, was a significant milestone in our understanding of particle physics.
Measuring the Mass of a Beta Particle: Experimental Techniques
Precise measurements of the electron's mass (and hence the beta particle's mass) have relied on several experimental techniques:
-
Thomson's Experiment: J.J. Thomson's famous experiment using cathode rays determined the charge-to-mass ratio (e/m) of the electron. Subsequent experiments determined the charge of the electron, allowing the calculation of its mass.
-
Millikan's Oil Drop Experiment: Robert Millikan's oil drop experiment provided a precise measurement of the elementary charge (e). This, combined with Thomson's e/m value, gave a highly accurate determination of the electron's mass.
-
Modern Techniques: Today, even more precise measurements of the electron's mass are obtained using advanced techniques like Penning traps, which can precisely measure the cyclotron frequency of an electron in a magnetic field.
Conclusion: A Tiny Particle, a Vast Impact
The seemingly insignificant mass of the beta particle belies its importance in the vast tapestry of nuclear physics and beyond. Its role in beta decay, a fundamental nuclear process, impacts our understanding of nuclear stability, stellar evolution, and even medical applications. The precise measurement of its mass has been a landmark achievement in physics, leading to further advancements in our knowledge of the universe at its most fundamental levels. From the intricacies of nuclear reactions to the applications in medicine and astrophysics, the beta particle's mass continues to be a crucial piece of the puzzle in unraveling the mysteries of the cosmos. Further research into this tiny particle will undoubtedly continue to refine our understanding of the universe and its fundamental forces.
Latest Posts
Latest Posts
-
How Do You Find The Boiling Point Of A Solution
Apr 02, 2025
-
Balance Equation Fes2 O2 Fe2o3 So2
Apr 02, 2025
-
Which Is Not A Physical Property
Apr 02, 2025
-
Australia Is The Worlds Leading Producer Of
Apr 02, 2025
-
Geometric Mean Of 8 And 18
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Of A Beta Particle . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
