Rank The Following Compounds In Order Of Decreasing Acidity
News Leon
Mar 30, 2025 · 7 min read
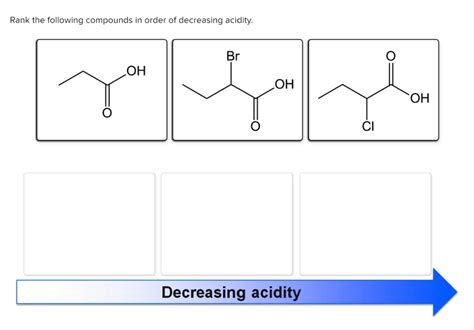
Table of Contents
Ranking Compounds by Decreasing Acidity: A Comprehensive Guide
Determining the relative acidity of different compounds is a fundamental concept in organic chemistry. Acidity is a measure of a compound's ability to donate a proton (H⁺). Several factors influence a compound's acidity, including the stability of the conjugate base formed after proton donation. This article will explore these factors and provide a comprehensive guide to ranking various compounds in order of decreasing acidity. We will delve into the underlying principles and provide numerous examples to solidify your understanding.
Factors Affecting Acidity
Before we delve into ranking specific compounds, let's review the key factors that determine a molecule's acidity:
1. Electronegativity:
The more electronegative an atom is, the better it can stabilize a negative charge. Therefore, compounds with more electronegative atoms directly bonded to the acidic proton will be more acidic. For example, consider HF, HCl, HBr, and HI. Fluorine is the most electronegative, followed by chlorine, bromine, and iodine. Thus, HF is the strongest acid, and HI is the weakest.
2. Inductive Effect:
Electron-withdrawing groups (EWGs) stabilize the negative charge on the conjugate base by pulling electron density away from the negatively charged atom. This stabilization increases the acidity of the compound. Conversely, electron-donating groups (EDGs) destabilize the negative charge, decreasing acidity. The strength of the inductive effect diminishes with distance from the acidic proton.
3. Resonance:
Resonance is a powerful stabilizing factor. If the conjugate base can delocalize the negative charge through resonance, the acidity of the parent compound will be significantly enhanced. The more resonance structures possible, the more stable the conjugate base, and hence the stronger the acid. Carboxylic acids are excellent examples of this, as the negative charge on the carboxylate ion is delocalized over two oxygen atoms.
4. Hybridization:
The hybridization of the atom bearing the negative charge influences its stability. More s-character in the hybrid orbital leads to greater electronegativity and better stabilization of the negative charge. For example, a sp hybridized carbon is more electronegative than an sp² hybridized carbon, which in turn is more electronegative than an sp³ hybridized carbon. This explains the trend in acidity of alkynes, alkenes, and alkanes.
5. Size and Steric Effects:
Larger atoms can better accommodate the negative charge due to their greater size and lower charge density. This effect is particularly noticeable in comparing the acidity of haloacids (HF, HCl, HBr, HI). Steric effects can also influence acidity by affecting the stability of the conjugate base. Bulky groups near the negatively charged atom can hinder solvation and destabilize the conjugate base, reducing acidity.
Ranking Examples and Explanations
Let's now put these principles into practice by ranking several sets of compounds in order of decreasing acidity:
Example 1: Alcohols vs. Carboxylic Acids
Consider the following compounds: ethanol (CH₃CH₂OH) and acetic acid (CH₃COOH).
- Acetic Acid (CH₃COOH) > Ethanol (CH₃CH₂OH)
Acetic acid is significantly more acidic than ethanol due to the resonance stabilization of its conjugate base (acetate ion). The negative charge on the acetate ion is delocalized over two oxygen atoms, making it much more stable than the ethoxide ion, which lacks resonance stabilization.
Example 2: Haloalkanes
Compare the acidity of the following haloalkanes: chloromethane (CH₃Cl), bromomethane (CH₃Br), and iodomethane (CH₃I).
- Bromomethane (CH₃Br) > Chloromethane (CH₃Cl) > Iodomethane (CH₃I)
While halogens are electronegative and exert an inductive effect, the effect is nuanced here. The inductive effect is stronger for smaller halogens, resulting in greater acidity. However, the size of the halogen also plays a role. While chlorine exerts a stronger inductive effect, the larger size of bromine and iodine allows better dispersal of the negative charge, resulting in a slight increase in acidity.
Example 3: Substituted Benzoic Acids
Let's rank the following substituted benzoic acids in order of decreasing acidity: benzoic acid (C₆H₅COOH), p-nitrobenzoic acid (p-NO₂C₆H₄COOH), and p-methoxybenzoic acid (p-CH₃OC₆H₄COOH).
- p-Nitrobenzoic acid (p-NO₂C₆H₄COOH) > Benzoic acid (C₆H₅COOH) > p-Methoxybenzoic acid (p-CH₃OC₆H₄COOH)
The nitro group (-NO₂) is a strong electron-withdrawing group, significantly stabilizing the conjugate base through resonance and the inductive effect. This leads to increased acidity. The methoxy group (-OCH₃) is an electron-donating group that destabilizes the conjugate base, decreasing acidity.
Example 4: Phenol vs. Cyclohexanol
Compare the acidity of phenol (C₆H₅OH) and cyclohexanol (C₆H₁₁OH).
- Phenol (C₆H₅OH) > Cyclohexanol (C₆H₁₁OH)
Phenol is significantly more acidic than cyclohexanol due to resonance stabilization. The negative charge on the phenoxide ion is delocalized into the aromatic ring, resulting in a much more stable conjugate base compared to the cyclohexoxide ion.
Example 5: Carboxylic Acids vs. Alcohols vs. Amides
Let's arrange these functional groups in order of decreasing acidity: carboxylic acid, alcohol, amide.
- Carboxylic Acid > Alcohol > Amide
Carboxylic acids are the most acidic due to the resonance stabilization of their conjugate base (carboxylate ion). Alcohols are less acidic than carboxylic acids due to the lack of resonance stabilization in their conjugate base (alkoxide ion). Amides are the least acidic of these three because the nitrogen lone pair donates electron density to the carbonyl oxygen, further destabilizing the conjugate base.
Example 6: Alpha-Substituted Acetic Acids
Consider the following alpha-substituted acetic acids: acetic acid (CH₃COOH), chloroacetic acid (ClCH₂COOH), and dichloroacetic acid (Cl₂CHCOOH).
- Dichloroacetic acid (Cl₂CHCOOH) > Chloroacetic acid (ClCH₂COOH) > Acetic acid (CH₃COOH)
The inductive effect of the chlorine atoms significantly increases the acidity. Two chlorine atoms exert a stronger inductive effect than one, thus dichloroacetic acid is more acidic than chloroacetic acid, which is more acidic than acetic acid.
Example 7: Comparing Different Types of Acids
Let's rank the following acids in order of decreasing acidity: hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and acetic acid (CH₃COOH).
- Hydrochloric acid (HCl) > Sulfuric acid (H₂SO₄) > Acetic acid (CH₃COOH)
Hydrochloric acid is a strong acid, completely dissociating in water. Sulfuric acid is also a strong acid, although less so than HCl. Acetic acid is a weak acid, meaning it only partially dissociates in water. The difference in acidity arises from the differences in bond strength and the stability of the conjugate bases.
Example 8: Hydrogen Halides
Rank the following hydrogen halides in decreasing acidity: HF, HCl, HBr, HI.
- HI > HBr > HCl > HF
Despite the high electronegativity of fluorine, HI is the strongest acid. This is because the large size of the iodine atom allows better accommodation of the negative charge on the iodide ion, leading to increased stability and greater acidity. The bond strength is also a significant factor; the H-I bond is the weakest, making it easier to break and donate a proton.
Advanced Considerations
While the above factors provide a good framework for understanding and predicting relative acidity, there are additional considerations in more complex scenarios:
-
Solvent Effects: The solvent in which the acid is dissolved can significantly impact its acidity. Polar protic solvents, such as water, can stabilize the conjugate base through hydrogen bonding, increasing the acidity.
-
Intramolecular Hydrogen Bonding: Intramolecular hydrogen bonding can stabilize the conjugate base and increase acidity.
-
Steric Hindrance: Bulky substituents near the acidic proton can hinder proton donation, reducing acidity.
Conclusion
Predicting the relative acidity of compounds requires a careful consideration of several factors. By understanding the interplay of electronegativity, inductive effects, resonance, hybridization, size, steric effects, and solvent interactions, you can accurately rank compounds according to their acidic strength. This detailed guide provides a robust foundation for tackling acidity problems in organic chemistry. Remember to always analyze the specific structural features of each compound to accurately assess its acidity. Practice is key to mastering this crucial concept.
Latest Posts
Latest Posts
-
What Is The Percent Of 13 20
Apr 01, 2025
-
If A Transversal Intersects Two Parallel Lines Then
Apr 01, 2025
-
State Any Two Effects Of Force
Apr 01, 2025
-
How Many Electrons Does Sodium Have In Its Outer Shell
Apr 01, 2025
-
Dense Irregular Connective Tissue Will Be Found In The
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Rank The Following Compounds In Order Of Decreasing Acidity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
