Photosynthesis Is A Physical Or Chemical Change
News Leon
Mar 27, 2025 · 6 min read
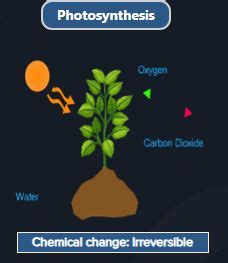
Table of Contents
Photosynthesis: A Chemical Change Fueling Life
Photosynthesis, the remarkable process by which green plants and certain other organisms convert light energy into chemical energy, is a fundamental pillar of life on Earth. But is this incredible transformation a physical change or a chemical change? The answer, definitively, is chemical change. While physical changes alter the form or appearance of a substance without changing its chemical composition, chemical changes involve the rearrangement of atoms and the formation of new substances. Photosynthesis, with its intricate molecular transformations, falls squarely into the latter category.
Understanding the Difference Between Physical and Chemical Changes
Before delving into the specifics of photosynthesis, let's establish a clear understanding of the distinction between physical and chemical changes.
Physical changes are reversible alterations that don't affect the chemical identity of a substance. Examples include:
- Melting ice: Ice (solid water) transforms into liquid water, but the chemical composition remains H₂O. The change is easily reversed by freezing.
- Boiling water: Liquid water turns into water vapor (steam), again without altering the chemical formula. Condensation reverses this process.
- Dissolving sugar in water: Sugar dissolves, forming a solution, but the sugar molecules remain intact. Evaporation of the water recovers the sugar.
Chemical changes, on the other hand, are irreversible transformations that result in the formation of new substances with different chemical properties. These changes are often accompanied by observable signs such as:
- Color change: A change in color often indicates a chemical reaction.
- Gas production: The release of gas (bubbles) is a hallmark of many chemical reactions.
- Temperature change: Reactions can either release heat (exothermic) or absorb heat (endothermic).
- Precipitate formation: The formation of a solid from a solution is another key indicator.
- Irreversibility: Chemical changes are generally not easily reversed.
Photosynthesis exhibits several of these indicators, clearly establishing it as a chemical change.
The Chemical Equation of Photosynthesis: A Molecular Transformation
Photosynthesis is a complex multi-step process, but its overall chemical equation neatly summarizes the transformation:
6CO₂ + 6H₂O + Light Energy → C₆H₁₂O₆ + 6O₂
This equation reveals the core of the chemical change:
- Reactants: Carbon dioxide (CO₂), water (H₂O), and light energy are the inputs.
- Products: Glucose (C₆H₁₂O₆), a simple sugar, and oxygen (O₂) are the outputs.
Notice that the atoms are rearranged. The carbon atoms from carbon dioxide combine with hydrogen atoms from water to form glucose. Oxygen atoms from water are released as oxygen gas. This rearrangement of atoms, the hallmark of a chemical reaction, is the defining characteristic of photosynthesis.
The Role of Chlorophyll and Light Energy
The process doesn't occur spontaneously. It requires chlorophyll, a green pigment found in chloroplasts within plant cells. Chlorophyll absorbs light energy, specifically in the red and blue regions of the visible spectrum, which is then used to drive the chemical reactions. This energy absorption is a crucial step in initiating the chemical transformation. Without light energy, the reaction wouldn't proceed.
The light-dependent reactions of photosynthesis involve the excitation of electrons in chlorophyll molecules by light energy. These excited electrons are then passed along an electron transport chain, generating ATP (adenosine triphosphate) and NADPH, which are energy-carrying molecules. These molecules then power the light-independent reactions.
Light-Independent Reactions (Calvin Cycle): Building Glucose
The light-independent reactions, also known as the Calvin cycle, use the ATP and NADPH generated in the light-dependent reactions to convert carbon dioxide into glucose. This intricate series of chemical reactions involves the fixation of carbon dioxide, the reduction of carbon dioxide molecules to a carbohydrate (glucose), and the regeneration of RuBP (ribulose-1,5-bisphosphate), a five-carbon sugar.
These chemical processes lead to the creation of glucose, a complex organic molecule with entirely different properties compared to the reactants. This clearly highlights the irreversible nature of the chemical transformation. The formation of a completely new substance, glucose, is a quintessential characteristic of a chemical change.
Evidence Supporting Photosynthesis as a Chemical Change
Several lines of evidence strongly support the classification of photosynthesis as a chemical change:
- Formation of new substances: The production of glucose and oxygen, substances with distinctly different chemical properties from carbon dioxide and water, undeniably proves the creation of new chemical entities. This is a definitive characteristic of a chemical reaction.
- Energy transformation: Photosynthesis converts light energy into chemical energy stored in the bonds of glucose molecules. This energy transformation is a hallmark of chemical processes. Energy is neither created nor destroyed; it is transformed from one form to another.
- Irreversibility: While the glucose produced can be broken down through respiration, the overall process of photosynthesis is not easily reversed. The simple reversion of conditions—removing light, for instance—will not restore the original reactants.
- Observable changes: Although subtle, observable changes such as the increased biomass of a plant over time, resulting from the accumulation of glucose, indirectly demonstrates the chemical process at play.
Misconceptions about Photosynthesis and Physical Changes
It's crucial to address common misconceptions that might blur the line between physical and chemical changes in the context of photosynthesis:
- Water absorption: Plants absorb water through their roots. This is a physical process of water movement, not a chemical transformation of the water molecule itself. The water molecules participate in the chemical reactions of photosynthesis, but the initial absorption is a separate physical phenomenon.
- Light absorption: Chlorophyll absorbs light. While crucial for initiating the process, light absorption itself is a physical process of energy transfer, not a chemical reaction. The subsequent use of this energy to drive the chemical reactions is where the chemical change occurs.
- Gas exchange: The release of oxygen and uptake of carbon dioxide are physical processes of gas exchange. However, the transformation of these gases into new molecules (glucose and water) during the photosynthetic process is a chemical change.
The Significance of Photosynthesis: A Chemical Foundation for Life
Photosynthesis is not merely a chemical process; it is the cornerstone of life on Earth. It is the primary source of energy for most ecosystems, forming the base of the food chain. The oxygen produced during photosynthesis is essential for the respiration of most living organisms. Without this remarkable chemical transformation, life as we know it would be impossible. Understanding photosynthesis as a chemical change is fundamental to understanding the chemistry of life itself. The detailed chemical mechanisms, the energy transfers, and the formation of entirely new compounds underscore the chemical nature of this critically important process. This comprehensive view empowers a deeper appreciation of the complexity and importance of photosynthesis in sustaining life on our planet.
Further Exploration: Deeper Dive into the Chemical Mechanisms
The explanation above provides a general overview. The detailed mechanisms of photosynthesis are far more complex, involving intricate enzyme-catalyzed reactions, electron transport chains, and proton gradients. Further investigation into these areas will reveal a deeper appreciation for the sophisticated chemical processes that underpin this fundamental life process. Researching specific enzymes involved, studying the detailed steps within the Calvin cycle, and exploring the various types of photosynthesis found in different organisms can greatly enhance understanding.
Conclusion: Photosynthesis – An Irreversible Chemical Marvel
In conclusion, photosynthesis is unequivocally a chemical change. The rearrangement of atoms, the formation of new substances (glucose and oxygen), the energy transformation, and the irreversibility of the process all point to a fundamental chemical transformation. Understanding the chemical nature of photosynthesis is vital for appreciating its profound impact on life on Earth and for developing new technologies and strategies for addressing environmental challenges, particularly those related to climate change and sustainable energy. The chemical reactions involved in photosynthesis are a testament to the incredible complexity and beauty of the natural world.
Latest Posts
Latest Posts
-
Evaporation Is A Physical Change True Or False
Mar 30, 2025
-
The Elongation Of The Leading Strand During Dna Synthesis
Mar 30, 2025
-
Which Of The Following Is A Closed System
Mar 30, 2025
-
Authorization Letter For Pick Up Documents
Mar 30, 2025
-
How Many Valence Electrons In Cesium
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Photosynthesis Is A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
