Which Of The Following Is A Closed System
News Leon
Mar 30, 2025 · 5 min read
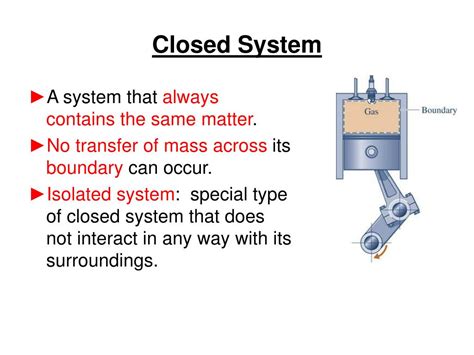
Table of Contents
Which of the Following is a Closed System? Understanding System Types in Physics and Beyond
The concept of a "closed system" is fundamental across various scientific disciplines, from physics and chemistry to ecology and even economics. Understanding what constitutes a closed system is crucial for accurate modeling, prediction, and interpretation of phenomena within these fields. This article will delve deep into the definition of a closed system, contrasting it with open and isolated systems, and providing numerous examples to solidify your understanding. We'll also explore the practical implications of classifying systems and the challenges in perfectly achieving a truly closed system in the real world.
Defining a Closed System
A closed system, in its simplest form, is a system that exchanges energy with its surroundings but not matter. This means energy, in the form of heat or work, can flow in and out of the system, but the amount of matter within the system remains constant. Think of it like a sealed container: energy can pass through the walls (e.g., heat radiating out), but no particles enter or leave.
This crucial distinction – the exchange of energy but not matter – is the defining characteristic of a closed system. It's this limitation on matter transfer that sets it apart from other types of systems.
Closed System vs. Open and Isolated Systems
To fully grasp the concept of a closed system, it's vital to contrast it with open and isolated systems:
Open Systems
Open systems exchange both energy and matter with their surroundings. They are characterized by a free flow of both energy and matter across their boundaries. Think of a boiling pot of water on a stove: heat energy is added from the stove (energy exchange), and water vapor escapes into the air (matter exchange). Biological organisms are prime examples of open systems; they constantly exchange energy and matter with their environments through respiration, nutrient uptake, and waste elimination.
Isolated Systems
Isolated systems are the most restrictive. They exchange neither energy nor matter with their surroundings. These are theoretical constructs, rarely perfectly achievable in reality. The universe as a whole is often considered (though debated) to be an isolated system. In practice, scientists attempt to create conditions closely approximating an isolated system through meticulous experimental design, often involving highly insulated containers or chambers.
Examples of Closed Systems: Real-World Applications
While perfect closed systems are challenging to create, many systems in the real world approximate this condition, offering valuable insights into various phenomena. Let's examine some key examples:
1. A Sealed Thermos Flask
A classic example is a sealed thermos flask containing hot coffee. Heat energy is slowly lost to the surroundings over time through radiation and conduction, but no coffee (matter) escapes or enters the flask. This approximates a closed system, allowing us to study the principles of heat transfer and cooling rates.
2. A Sealed Chemical Reaction Vessel
In chemistry, a sealed reaction vessel containing reactants undergoing a chemical reaction is another example. Energy in the form of heat might be absorbed or released during the reaction, but the mass of the system (the total amount of reactants and products) remains constant, assuming the container is perfectly sealed. This is important for determining reaction yields and studying reaction kinetics.
3. The Earth's Atmosphere (with caveats)
In some contexts, the Earth's atmosphere can be approximated as a closed system. While energy exchange with space (solar radiation and infrared radiation) is significant, the net exchange of matter with space is relatively small compared to the total atmospheric mass. However, this is a simplification. The Earth-atmosphere system is more accurately considered a nearly-closed system. Meteorites adding mass and gases escaping to space are examples of matter exchange, albeit on a smaller scale.
4. A Cylinder with a Frictionless Piston
In thermodynamics, a cylinder filled with a gas and sealed with a frictionless piston is a useful model of a closed system. Work can be done on or by the gas (energy exchange) as the piston moves, but the amount of gas within the cylinder (matter) remains constant. This system allows for the study of thermodynamic processes like adiabatic expansion and compression.
5. A Car Engine (Simplified Model)
For certain analyses, a simplified model of a car engine can be treated as a closed system. While fuel and exhaust gases are exchanged, focusing solely on the thermodynamic processes within the cylinders, ignoring the flow of fuel and exhaust, can provide valuable insights into engine efficiency and performance. This is a simplification, as a car engine is inherently an open system.
Challenges in Creating Truly Closed Systems
Creating a perfectly closed system is virtually impossible. Even seemingly sealed containers have microscopic pores, allowing for the slow exchange of gases. Similarly, radiation can always transfer energy, regardless of the material used for containment. Therefore, in practice, we often deal with systems that are nearly closed or are considered closed for the purposes of simplification or modeling.
The Importance of System Classification
The classification of systems—as open, closed, or isolated—is critical for several reasons:
- Accurate Modeling: Different types of systems require different mathematical models and equations. A model appropriate for a closed system would be inappropriate for an open system.
- Prediction and Simulation: Understanding the type of system allows for more accurate predictions of its behavior under different conditions. Simulations based on incorrect system classification will yield inaccurate results.
- Experimental Design: Researchers must account for system type when designing experiments. Controlling for energy and matter exchange is crucial for reliable results.
Conclusion: Context is Key
The classification of a system as open, closed, or isolated is heavily dependent on the context and the specific phenomenon under investigation. What might be considered a closed system in one scenario could be an open system in another. Careful consideration of the system's boundaries and the interactions with its surroundings is paramount to accurate system classification and subsequent analysis. Understanding this distinction is fundamental to applying scientific principles and creating robust models across various fields. By appreciating the nuances of system types, we can better understand the complex interactions that shape the world around us.
Latest Posts
Latest Posts
-
Do Lysosomes Have A Double Membrane
Apr 01, 2025
-
Which Of The Following Is True Of B Cells
Apr 01, 2025
-
How Many Parents Are Involved In Asexual Reproduction
Apr 01, 2025
-
What Is The Order Of The Breakdown Products Of Hemoglobin
Apr 01, 2025
-
What Muscle Subdivides The Ventral Body Cavity
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Closed System . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
