Oxidation Number Of S In H2so4
News Leon
Mar 19, 2025 · 7 min read
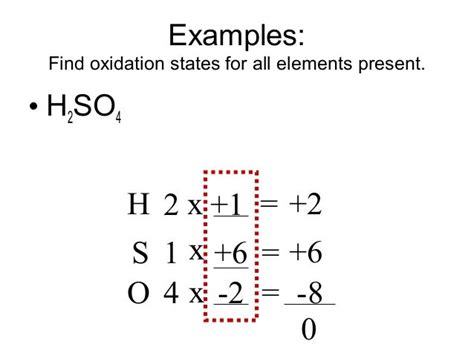
Table of Contents
Determining the Oxidation Number of Sulfur in H₂SO₄
Sulfuric acid (H₂SO₄), a cornerstone chemical in countless industrial processes, presents a straightforward yet instructive example for understanding oxidation numbers. This article will delve deeply into the method for calculating the oxidation number of sulfur (S) in H₂SO₄, exploring the underlying principles and providing a comprehensive understanding of this fundamental concept in chemistry.
Understanding Oxidation Numbers
Before tackling the specific case of H₂SO₄, let's establish a firm grasp of what oxidation numbers represent. The oxidation number, also known as the oxidation state, is a number assigned to an atom in a chemical compound that represents the hypothetical charge the atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool for understanding redox reactions (reduction-oxidation reactions), where electron transfer occurs between atoms. While not a true charge, it helps track electron movement and predict chemical behavior.
Key Rules for Assigning Oxidation Numbers:
-
Free elements: The oxidation number of an atom in its elemental form is always zero (e.g., O₂ has an oxidation number of 0 for each oxygen atom).
-
Monatomic ions: The oxidation number of a monatomic ion equals its charge (e.g., Na⁺ has an oxidation number of +1, Cl⁻ has an oxidation number of -1).
-
Hydrogen: Hydrogen typically has an oxidation number of +1, except in metal hydrides (e.g., NaH), where it is -1.
-
Oxygen: Oxygen usually has an oxidation number of -2, except in peroxides (e.g., H₂O₂) where it is -1, and in compounds with fluorine (e.g., OF₂), where it is +2.
-
Group 1 and Group 2 elements: Group 1 elements (alkali metals) always have an oxidation number of +1, and Group 2 elements (alkaline earth metals) always have an oxidation number of +2.
-
The sum of oxidation numbers: In a neutral molecule, the sum of the oxidation numbers of all atoms equals zero. In a polyatomic ion, the sum of the oxidation numbers equals the charge of the ion.
Calculating the Oxidation Number of Sulfur in H₂SO₄
Now, let's apply these rules to determine the oxidation number of sulfur in sulfuric acid (H₂SO₄).
Step 1: Assign known oxidation numbers:
- Hydrogen (H): Following rule 3, each hydrogen atom has an oxidation number of +1. Since there are two hydrogen atoms, their total contribution is 2(+1) = +2.
- Oxygen (O): Following rule 4, each oxygen atom has an oxidation number of -2. With four oxygen atoms, their total contribution is 4(-2) = -8.
Step 2: Set up the equation:
Let 'x' represent the oxidation number of sulfur (S). The sum of the oxidation numbers of all atoms in the neutral H₂SO₄ molecule must equal zero (rule 6). Therefore, we can write the equation:
(+2) + x + (-8) = 0
Step 3: Solve for x:
Simplifying the equation:
x - 6 = 0
x = +6
Conclusion: The oxidation number of sulfur (S) in H₂SO₄ is +6.
Implications of the +6 Oxidation State of Sulfur
The +6 oxidation state of sulfur in H₂SO₄ is the highest oxidation state sulfur can achieve. This high oxidation state reflects the strong oxidizing power of sulfuric acid, particularly in its concentrated form. This oxidizing power is manifested in several key applications:
Industrial Applications of Sulfuric Acid's Oxidizing Power
-
Metal Refining: Concentrated sulfuric acid is used in the refining of various metals due to its ability to dissolve and oxidize many metals, separating them from impurities.
-
Chemical Synthesis: It serves as a potent oxidizing agent in numerous chemical synthesis reactions, enabling the production of various organic and inorganic compounds. Its role is often not merely as an acid but as an active participant in the oxidation process.
-
Battery Production: Sulfuric acid is the electrolyte in lead-acid batteries, where its ability to facilitate electron transfer between the lead plates is crucial for the battery's operation. The oxidation and reduction reactions involving lead and sulfuric acid provide the electrical energy.
-
Petroleum Refining: In the refining of petroleum, sulfuric acid acts as a catalyst and an oxidizing agent in various processes, contributing to the production of gasoline and other petroleum products. Its role here includes the removal of impurities and the conversion of certain hydrocarbon fractions.
-
Fertilizer Production: The production of phosphate fertilizers often utilizes sulfuric acid to convert phosphate rock into a form that plants can more readily absorb. This conversion involves chemical reactions where sulfuric acid plays both an acidic and oxidizing role.
The Role of Oxidation State in Redox Reactions
Understanding the oxidation state of sulfur in H₂SO₄ is critical for comprehending its involvement in redox reactions. When H₂SO₄ acts as an oxidizing agent, the sulfur atom undergoes a reduction, its oxidation state decreasing. Conversely, when it is reduced, the sulfur atom's oxidation state increases. This change in oxidation state is the defining characteristic of redox reactions.
For example, in the reaction of concentrated sulfuric acid with a metal like copper:
Cu(s) + 2H₂SO₄(aq) → CuSO₄(aq) + SO₂(g) + 2H₂O(l)
The copper is oxidized (loses electrons), while the sulfur in some of the sulfuric acid is reduced (gains electrons) from a +6 oxidation state to a +4 oxidation state in sulfur dioxide (SO₂). This demonstrates the simultaneous oxidation and reduction characteristic of redox reactions.
Beyond H₂SO₄: Oxidation States of Sulfur
Sulfur exhibits a remarkable range of oxidation states, showcasing its versatility in chemical bonding. This flexibility stems from its electronic configuration, allowing it to readily gain or lose electrons. Some key examples include:
-
Sulfides (-2): In compounds like hydrogen sulfide (H₂S), sulfur has an oxidation state of -2. This is the lowest oxidation state commonly observed.
-
Sulfites (+4): In sulfites (SO₃²⁻), sulfur displays an oxidation state of +4.
-
Thiosulfates (+2): In thiosulfates (S₂O₃²⁻), sulfur exhibits two different oxidation states: +2 and -2.
-
Elemental Sulfur (0): Elemental sulfur (S₈) exists in its native form with an oxidation state of 0.
The variety of oxidation states attainable by sulfur contributes to the wide array of chemical compounds it forms and the diversity of its roles in both natural and industrial processes. Understanding its oxidation state in each compound is essential for predicting chemical reactivity and designing chemical reactions effectively.
Advanced Concepts and Further Exploration
The concept of oxidation numbers extends beyond simple inorganic compounds. It's applicable to complex organic molecules, coordination compounds, and even materials science. Further exploration into these areas reveals the power and versatility of oxidation numbers as a predictive tool in chemistry:
-
Organic Chemistry: Assigning oxidation numbers to carbon atoms in organic molecules is crucial for understanding oxidation reactions such as combustion and the mechanisms of various organic transformations. For instance, the oxidation of alcohols to aldehydes or ketones involves a change in the oxidation state of the carbon atom.
-
Coordination Chemistry: In coordination complexes, the oxidation state of the central metal ion is pivotal in determining the complex's properties and reactivity. The ligands surrounding the metal ion also influence the overall oxidation state and behavior of the complex.
-
Electrochemistry: Oxidation numbers form the foundation of electrochemical processes. Electrochemical cells rely on the transfer of electrons, which directly relates to changes in oxidation states of the participating species. Understanding oxidation states is vital for predicting cell potentials and designing efficient electrochemical systems.
-
Materials Science: The oxidation states of elements in materials significantly impact their physical and chemical properties. For example, the oxidation state of iron in iron oxides determines their magnetic and catalytic properties. Controlling the oxidation states of elements is crucial in materials design and synthesis.
Understanding the oxidation number of sulfur in H₂SO₄ provides a stepping stone towards a deeper appreciation of redox chemistry, its implications across various fields of science, and the power of this fundamental concept in predicting and understanding chemical behavior. By mastering these basic principles, you'll gain a robust foundation for more advanced studies in chemistry and related disciplines.
Latest Posts
Latest Posts
-
Homogeneous Mixtures Are Also Known As
Mar 19, 2025
-
Locus Of Points Equidistant From A Point And A Circle
Mar 19, 2025
-
Reproduction Is Not Essential For The Survival Of An Individual
Mar 19, 2025
-
What Is The Molecular Mass Of H3po4
Mar 19, 2025
-
Why A Cells Size Is Limited
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of S In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
