What Is The Molecular Mass Of H3po4
News Leon
Mar 19, 2025 · 5 min read
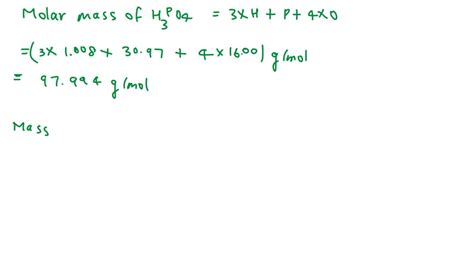
Table of Contents
What is the Molecular Mass of H3PO4? A Deep Dive into Phosphoric Acid
Phosphoric acid, with its chemical formula H₃PO₄, is a crucial compound in various industrial and biological processes. Understanding its molecular mass is fundamental to many applications, from fertilizer production to biochemical research. This comprehensive article will delve into the calculation of H₃PO₄'s molecular mass, exploring the underlying principles and providing a detailed breakdown of the process. We will also discuss the significance of molecular mass in different contexts and touch upon related concepts.
Understanding Molecular Mass
Before calculating the molecular mass of H₃PO₄, let's define the term. Molecular mass, also known as molecular weight, represents the mass of a molecule. It's expressed in atomic mass units (amu) or Daltons (Da). Unlike atomic mass, which refers to a single atom, molecular mass considers the combined mass of all atoms within a molecule. This mass is crucial in various chemical calculations, including stoichiometry, determining concentrations, and understanding reaction rates.
Calculating the Molecular Mass of H3PO4
The calculation of H₃PO₄'s molecular mass involves determining the atomic mass of each element present in the molecule (hydrogen, phosphorus, and oxygen) and then summing them up according to the number of atoms of each element. Let's break down the process step-by-step:
1. Atomic Masses of Constituent Elements
We need the standard atomic masses of hydrogen (H), phosphorus (P), and oxygen (O). These values are typically found on the periodic table and are often rounded for simplicity in calculations:
- Hydrogen (H): Approximately 1.008 amu
- Phosphorus (P): Approximately 30.97 amu
- Oxygen (O): Approximately 16.00 amu
2. Counting Atoms in the H3PO4 Molecule
The chemical formula H₃PO₄ tells us the number of atoms of each element present in one molecule of phosphoric acid:
- Hydrogen (H): 3 atoms
- Phosphorus (P): 1 atom
- Oxygen (O): 4 atoms
3. Calculating the Total Molecular Mass
Now, we multiply the atomic mass of each element by the number of atoms of that element in the molecule and then sum the results:
- Mass of Hydrogen: 3 atoms × 1.008 amu/atom = 3.024 amu
- Mass of Phosphorus: 1 atom × 30.97 amu/atom = 30.97 amu
- Mass of Oxygen: 4 atoms × 16.00 amu/atom = 64.00 amu
Total Molecular Mass of H₃PO₄: 3.024 amu + 30.97 amu + 64.00 amu = 97.994 amu
Therefore, the molecular mass of H₃PO₄ is approximately 97.99 amu (rounding to two decimal places).
Significance of Molecular Mass in Different Contexts
The precise determination of the molecular mass of H₃PO₄, and other compounds, is critical across diverse scientific and industrial fields:
1. Stoichiometry and Chemical Reactions
Molecular mass is essential for stoichiometric calculations. It allows us to determine the precise amounts of reactants needed for a chemical reaction and the expected amount of products formed. In the case of H₃PO₄ reactions, knowing its molecular mass ensures accurate calculations of reactant ratios and product yields.
2. Solution Chemistry and Concentration
When working with solutions of phosphoric acid, the molecular mass is crucial for calculating molarity (moles of solute per liter of solution). Molarity is a fundamental concept in chemistry, allowing for precise control over reaction conditions and concentration-dependent properties.
3. Biochemistry and Biological Systems
In biochemical research, understanding the molecular mass of H₃PO₄ is important because it plays a vital role in various biological processes. For instance, it's a component of ATP (adenosine triphosphate), the primary energy carrier in living cells. Knowing its mass facilitates studies involving energy metabolism and related pathways.
4. Fertilizer Production and Agricultural Applications
Phosphoric acid is a key ingredient in the production of phosphate fertilizers. Accurate knowledge of its molecular mass is essential for determining the correct proportions of other ingredients in fertilizer formulations, ensuring optimal nutrient delivery to crops.
5. Industrial Applications
H₃PO₄ finds widespread application in numerous industrial processes, including food processing (as a food additive), metal treatment (for rust removal), and the manufacturing of detergents. Precise calculations involving its molecular mass are crucial for maintaining quality control and optimizing production efficiency.
Beyond the Basic Calculation: Isotopes and Isotopic Abundance
The molecular mass calculation presented above uses the average atomic masses of the elements. However, elements exist as isotopes – atoms with the same number of protons but different numbers of neutrons. These isotopes have slightly different masses. To determine a highly precise molecular mass, the isotopic abundances of each element must be considered. This calculation would involve a weighted average of the molecular masses considering all isotopic variations of H, P, and O. While the average molecular mass is sufficient for most practical applications, highly precise scientific work may necessitate this more complex approach.
Related Concepts: Molar Mass and Avogadro's Number
The term molar mass is closely related to molecular mass. The molar mass of a substance is the mass of one mole of that substance. One mole contains Avogadro's number (approximately 6.022 × 10²³) of particles (atoms, molecules, ions, etc.). Therefore, the molar mass of H₃PO₄ is numerically equal to its molecular mass, but the units change from amu to grams per mole (g/mol). The molar mass of H₃PO₄ is approximately 97.99 g/mol. This concept is invaluable in chemical calculations involving large quantities of substances.
Conclusion: The Importance of Precision in Molecular Mass Calculations
The molecular mass of H₃PO₄, approximately 97.99 amu or 97.99 g/mol, is a fundamental piece of information with widespread applications. Its accurate determination is crucial for various scientific and industrial processes. While the basic calculation using average atomic masses provides a sufficiently accurate value for most purposes, the complexities of isotopic abundances underscore the importance of precision in certain contexts. Understanding the nuances of molecular mass calculation and its significance in different fields enhances our ability to utilize H₃PO₄ and other compounds effectively in various applications. This knowledge is critical for scientists, engineers, and anyone working with chemicals in any capacity.
Latest Posts
Latest Posts
-
How Did Hoover React To The Bonus Army
Mar 19, 2025
-
What Is The Electron Configuration Of Ti
Mar 19, 2025
-
I Wandered Lonely As Cloud Summary
Mar 19, 2025
-
What Forms The Backbone Of Dna
Mar 19, 2025
-
The Source Of Oxygen Produced During Photosynthesis Is
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of H3po4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
