What Is The Electron Configuration Of Ti
News Leon
Mar 19, 2025 · 6 min read
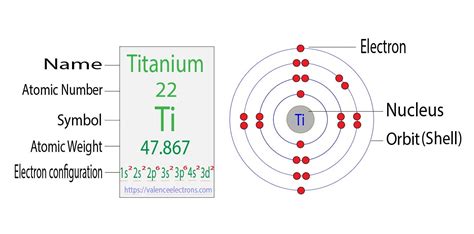
Table of Contents
What is the Electron Configuration of Ti? A Deep Dive into Titanium's Atomic Structure
Titanium (Ti), a lustrous transition metal with the atomic number 22, holds a significant place in various industries due to its unique properties: strength, lightweight nature, and corrosion resistance. Understanding its electron configuration is key to comprehending these properties and its chemical behavior. This article will explore the electron configuration of titanium in detail, examining its implications for chemical bonding, reactivity, and its overall place in the periodic table.
Understanding Electron Configuration
Before diving into titanium's specific configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates how an atom interacts with other atoms, forming chemical bonds and influencing its physical and chemical properties. It follows specific rules based on quantum mechanics and the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The Aufbau Principle
The Aufbau principle, also known as the building-up principle, states that electrons fill atomic orbitals in order of increasing energy levels. This means that lower energy levels are filled before higher energy levels. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, and so on.
Hund's Rule
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion, leading to a more stable configuration. Each orbital within a subshell is first filled with a single electron before any pairing occurs.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This means that each orbital can hold a maximum of two electrons, with opposite spins (+1/2 and -1/2).
Determining the Electron Configuration of Titanium (Ti)
Titanium has an atomic number of 22, meaning it has 22 protons and 22 electrons in a neutral atom. Using the Aufbau principle, we can systematically fill the orbitals to determine its electron configuration.
-
1s²: The first energy level (n=1) contains the 1s subshell, which can hold a maximum of two electrons. These are filled first.
-
2s²: Next, the 2s subshell (n=2, l=0) is filled with two electrons.
-
2p⁶: The 2p subshell (n=2, l=1) has three orbitals, each capable of holding two electrons, for a total of six electrons.
-
3s²: The 3s subshell (n=3, l=0) accommodates another two electrons.
-
3p⁶: The 3p subshell (n=3, l=1) holds six more electrons.
-
4s²: The 4s subshell (n=4, l=0) is filled with two electrons. Note: While the 3d subshell has a slightly higher energy than the 4s subshell, the 4s subshell fills first according to the Aufbau principle's general order.
-
3d²: Finally, the remaining two electrons fill the 3d subshell (n=3, l=2). This subshell can hold up to ten electrons.
Therefore, the complete electron configuration of titanium is: 1s²2s²2p⁶3s²3p⁶4s²3d².
Alternative Notation: Condensed Electron Configuration
A more concise way to represent the electron configuration is using the noble gas core notation. This involves using the symbol of the preceding noble gas (the element in the previous period with a full valence shell) followed by the remaining electrons. The noble gas preceding titanium is Argon (Ar), which has the electron configuration 1s²2s²2p⁶3s²3p⁶. Therefore, the condensed electron configuration of titanium is: [Ar] 4s²3d².
Implications of Titanium's Electron Configuration
Titanium's electron configuration directly impacts its chemical and physical properties:
Chemical Reactivity and Bonding
The two electrons in the 4s subshell and the two electrons in the 3d subshell are the valence electrons – the electrons involved in chemical bonding. Titanium readily loses these electrons to form stable ions, typically with a +4 oxidation state (Ti⁴⁺). However, it can also exhibit +2 and +3 oxidation states depending on the reaction conditions. This variable oxidation state contributes to titanium's diverse chemistry and its ability to form a wide range of compounds.
Metallic Bonding and Properties
The presence of valence electrons in both the 4s and 3d orbitals contributes to strong metallic bonding in titanium. These delocalized electrons create a "sea" of electrons that holds the titanium atoms together, accounting for its high strength, high melting point, and excellent electrical conductivity.
Transition Metal Characteristics
Titanium's partially filled 3d subshell classifies it as a transition metal. This incomplete d-subshell is responsible for many of titanium's characteristic properties, including its ability to form colored compounds and act as a catalyst in certain chemical reactions. The variable oxidation states also contribute to its catalytic activity.
Titanium's Importance in Various Applications
The unique combination of properties stemming from its electron configuration makes titanium highly valuable in several industrial applications:
-
Aerospace: Its high strength-to-weight ratio makes it ideal for aircraft and spacecraft components, where lightweight yet strong materials are crucial.
-
Biomedical Implants: Titanium's biocompatibility and corrosion resistance make it suitable for creating implants such as artificial joints and dental implants. Its inertness within the body minimizes rejection and adverse reactions.
-
Chemical Processing: Titanium's resistance to corrosion allows its use in handling corrosive chemicals and in high-temperature applications.
-
Sporting Goods: Its strength and lightness are exploited in the production of sporting equipment, such as bicycles and golf clubs.
Further Exploration: Excited States and Ionization Energies
While the ground state electron configuration detailed above is the most stable arrangement, titanium, like any atom, can exist in excited states. By absorbing energy, an electron can jump to a higher energy level, resulting in a different electron configuration. These excited states are often short-lived but play a significant role in spectroscopic analysis and chemical reactions.
The ionization energies of titanium also reflect its electron configuration. The energy required to remove the first electron (the first ionization energy) is relatively low compared to subsequent ionization energies. This indicates that the 4s electrons are more easily removed than the 3d electrons, which are held more tightly to the nucleus.
Conclusion: A Comprehensive Look at Titanium's Electron Configuration
The electron configuration of titanium, [Ar] 4s²3d², is not merely a collection of numbers and letters but a fundamental description of its atomic structure that determines its properties and behavior. Understanding this configuration helps explain its strength, lightweight nature, corrosion resistance, and its diverse applications in various fields. From the intricacies of chemical bonding to its significance in modern technology, titanium's electron configuration provides a crucial key to unlocking its remarkable versatility. Further exploration of excited states and ionization energies enhances this understanding, providing a deeper appreciation of this versatile transition metal and its place within the periodic table.
Latest Posts
Latest Posts
-
How Many Lines Of Symmetry Does A Rhombus Have
Mar 19, 2025
-
Which Of The Following Is A Hinge Joint
Mar 19, 2025
-
How Did The Treaty Of Versailles Help Cause Ww2
Mar 19, 2025
-
What Mountain Range Separates Europe And Asia
Mar 19, 2025
-
What Is 8 Percent As A Decimal
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Ti . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
