Homogeneous Mixtures Are Also Known As
News Leon
Mar 19, 2025 · 6 min read
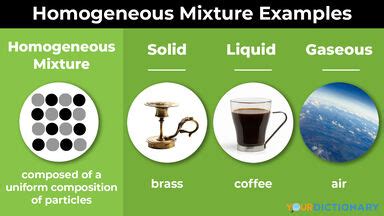
Table of Contents
- Homogeneous Mixtures Are Also Known As
- Table of Contents
- Homogeneous Mixtures: A Deep Dive into Solutions and Their Properties
- Defining Homogeneous Mixtures: A Uniform Composition
- Key Differences from Heterogeneous Mixtures
- Types of Homogeneous Mixtures: Exploring Solutions
- 1. Liquid Solutions: The Most Common Type
- 2. Gaseous Solutions: A Uniform Blend of Gases
- 3. Solid Solutions: Alloys and More
- Factors Affecting the Formation of Homogeneous Mixtures
- Properties of Homogeneous Mixtures
- Applications of Homogeneous Mixtures
- Conclusion: The Significance of Homogeneous Mixtures
- Latest Posts
- Latest Posts
- Related Post
Homogeneous Mixtures: A Deep Dive into Solutions and Their Properties
Homogeneous mixtures, also known as solutions, are ubiquitous in our daily lives. From the air we breathe to the blood flowing through our veins, these seemingly simple substances play a crucial role in various natural and man-made processes. Understanding their properties and characteristics is essential across numerous scientific disciplines, including chemistry, physics, and materials science. This comprehensive article will delve into the intricacies of homogeneous mixtures, exploring their defining features, examples, and practical applications.
Defining Homogeneous Mixtures: A Uniform Composition
The defining characteristic of a homogeneous mixture is its uniform composition. This means that the components of the mixture are evenly distributed throughout the entire sample. Regardless of the portion you examine, the ratio of constituents remains consistent. Unlike heterogeneous mixtures, where distinct phases or regions are visible (think of a salad with its various ingredients), homogeneous mixtures present a single, visually indistinguishable phase.
This uniformity extends to the molecular level. The particles of the solute (the substance being dissolved) are dispersed evenly among the particles of the solvent (the substance doing the dissolving) at a molecular or ionic level. This intimate mixing results in a solution with consistent properties throughout.
Key Differences from Heterogeneous Mixtures
To fully grasp the concept of a homogeneous mixture, it's essential to contrast it with its counterpart: a heterogeneous mixture. Here's a concise comparison:
| Feature | Homogeneous Mixture | Heterogeneous Mixture |
|---|---|---|
| Composition | Uniform throughout | Non-uniform, with distinct regions |
| Phases | Single phase | Two or more phases visible |
| Particle Size | Particles are smaller than 1 nm (ions, atoms, molecules) | Particle size varies; may be visible to the naked eye |
| Separation | Components cannot be easily separated by physical means | Components can be easily separated by physical means |
| Examples | Air, saltwater, sugar dissolved in water | Sand and water, oil and water, salad dressing |
Types of Homogeneous Mixtures: Exploring Solutions
Homogeneous mixtures are often referred to as solutions, a term that encompasses a wide array of mixtures based on the state of matter of the solvent and solute.
1. Liquid Solutions: The Most Common Type
Liquid solutions are by far the most prevalent type of homogeneous mixture. The solvent is a liquid, and the solute can be a solid, liquid, or gas. Examples abound:
-
Solid dissolved in liquid: Saltwater (sodium chloride dissolved in water), sugar water (sucrose dissolved in water), saline solution (sodium chloride in water used in medical settings). These are common examples that illustrate the dissolution process, where the solute particles are surrounded by solvent molecules.
-
Liquid dissolved in liquid: Alcohol in water (e.g., alcoholic beverages), vinegar (acetic acid in water). Miscibility, the ability of liquids to mix completely, is crucial in forming homogeneous liquid-liquid solutions.
-
Gas dissolved in liquid: Carbonated water (carbon dioxide dissolved in water), oxygen dissolved in water (essential for aquatic life). The solubility of gases in liquids is often temperature-dependent; increased temperature usually reduces gas solubility.
2. Gaseous Solutions: A Uniform Blend of Gases
Gaseous solutions are mixtures where both the solute and solvent are in the gaseous state. Air is the quintessential example, a homogeneous mixture of predominantly nitrogen, oxygen, argon, and trace amounts of other gases. The even distribution of these gases contributes to the consistent properties of air throughout the atmosphere.
3. Solid Solutions: Alloys and More
Solid solutions are less common but equally important. These solutions involve a solid solute dissolved in a solid solvent. Alloys are prime examples:
- Brass: A mixture of copper and zinc. The zinc atoms are dispersed throughout the copper lattice, altering the properties of the pure metal.
- Steel: A mixture of iron and carbon, with varying amounts of other elements. The carbon content dictates the properties of steel, ranging from soft and malleable to hard and strong.
Factors Affecting the Formation of Homogeneous Mixtures
Several factors influence the formation and stability of homogeneous mixtures:
-
Solubility: This refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. The solubility of a substance is determined by the intermolecular forces between the solute and solvent molecules. "Like dissolves like" is a useful rule of thumb: polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
-
Temperature: Increasing the temperature generally increases the solubility of solids in liquids, but it often decreases the solubility of gases in liquids. This is due to the increased kinetic energy of the particles at higher temperatures.
-
Pressure: Pressure has a more significant effect on the solubility of gases in liquids. Increasing pressure increases the solubility of gases, according to Henry's Law.
-
Concentration: This refers to the amount of solute dissolved in a given amount of solvent or solution. It can be expressed in various ways, including molarity, molality, and percent by mass.
Properties of Homogeneous Mixtures
Homogeneous mixtures possess several distinctive properties:
-
Uniform appearance: As mentioned earlier, the most defining feature is their uniform appearance; they have a single, visually indistinguishable phase.
-
Constant composition: The ratio of components remains consistent throughout the entire sample.
-
Filtrability: The components cannot be separated by simple filtration because the particle size of the solute is smaller than the pore size of the filter.
-
Consistent physical properties: Homogeneous mixtures possess consistent physical properties, such as density, boiling point, and refractive index, throughout the solution.
Applications of Homogeneous Mixtures
The applications of homogeneous mixtures are vast and span numerous fields:
-
Medicine: Many medications are administered as solutions, ensuring consistent drug delivery. Intravenous (IV) fluids are aqueous solutions that maintain electrolyte balance.
-
Food and beverage industry: Many processed foods and beverages are homogeneous mixtures. Soft drinks, juices, and sauces are examples.
-
Industrial processes: Chemical reactions often occur in solution form to enhance reactivity. Many industrial processes rely on homogeneous mixtures for efficient and controlled reactions.
-
Environmental science: Understanding the composition and behavior of homogeneous mixtures like air and water is crucial in environmental monitoring and pollution control.
Conclusion: The Significance of Homogeneous Mixtures
Homogeneous mixtures, or solutions, are fundamental to our understanding of chemistry and the world around us. Their uniform composition, predictable properties, and diverse applications make them essential in various scientific and technological fields. From the air we breathe to the sophisticated chemical reactions driving modern technologies, homogeneous mixtures are integral to countless processes that shape our lives. Continued research and advancements in our understanding of these mixtures will undoubtedly lead to further innovations and applications across a vast range of disciplines.
Latest Posts
Latest Posts
-
Which Of The Following Has The Higher Energy
Mar 20, 2025
-
What Happens To A Cell In A Hypertonic Solution
Mar 20, 2025
-
The Second Phase Of Mitosis Is Called
Mar 20, 2025
-
Henrys Law Constant Co2 In Water
Mar 20, 2025
-
What Is The Most Reactive Nonmetal
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Homogeneous Mixtures Are Also Known As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
