Oxidation Number Of Oxygen In H2o
News Leon
Mar 23, 2025 · 6 min read
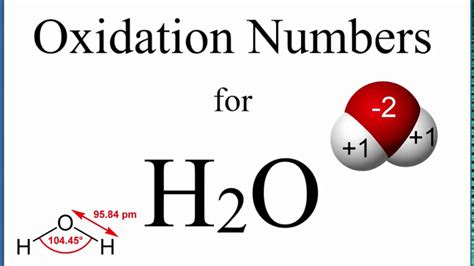
Table of Contents
Oxidation Number of Oxygen in H₂O: A Deep Dive
The seemingly simple molecule of water, H₂O, offers a fascinating entry point into the world of oxidation numbers. While the concept might seem daunting at first, understanding the oxidation number of oxygen in H₂O is crucial for grasping fundamental chemistry principles, including redox reactions and chemical bonding. This article will explore this topic in detail, providing a comprehensive understanding suitable for both beginners and those seeking a deeper dive.
What is an Oxidation Number?
Before delving into the specifics of oxygen in water, let's establish a clear understanding of oxidation numbers. An oxidation number, also known as an oxidation state, is a number assigned to an atom in a chemical compound that represents the number of electrons that atom has gained or lost compared to its neutral state. It's a bookkeeping tool that helps us track electron transfer in chemical reactions, particularly redox (reduction-oxidation) reactions.
It's important to note that oxidation numbers are not necessarily the actual charges on atoms; they are a formal assignment based on a set of rules. These rules help us consistently assign oxidation numbers, even in molecules where the bonding is complex and doesn't perfectly reflect a simple transfer of electrons.
Rules for Assigning Oxidation Numbers
Several rules guide the assignment of oxidation numbers. These rules are hierarchical; if a rule conflicts with another, the higher-priority rule takes precedence:
-
The oxidation number of an element in its free (uncombined) state is zero. For example, the oxidation number of O₂ (oxygen gas) is zero, and the oxidation number of Fe (iron) is zero.
-
The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ (sodium ion) is +1, and the oxidation number of Cl⁻ (chloride ion) is -1.
-
The oxidation number of hydrogen is +1, except when it is bonded to a less electronegative element (like alkali metals and alkaline earth metals), where it is -1. In most compounds, hydrogen has an oxidation number of +1 (e.g., in H₂O). However, in metal hydrides like NaH, hydrogen has an oxidation number of -1.
-
The oxidation number of oxygen is -2, except in peroxides (where it is -1) and in compounds with fluorine (where it can be positive). This is a crucial rule for understanding the oxidation number of oxygen in H₂O.
-
The sum of the oxidation numbers of all atoms in a neutral molecule is zero. This is a vital rule for checking your work and solving for unknown oxidation numbers.
-
The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. This rule is essential for working with polyatomic ions.
Determining the Oxidation Number of Oxygen in H₂O
Applying these rules to water (H₂O), we can determine the oxidation number of oxygen:
-
Hydrogen (H): Based on rule 3, the oxidation number of each hydrogen atom is +1. Since there are two hydrogen atoms, the total contribution from hydrogen is +2.
-
Oxygen (O): We need to find the oxidation number of oxygen (let's call it 'x').
-
Rule 5 Application: The sum of the oxidation numbers in a neutral molecule (H₂O) is zero. Therefore:
(+1) + (+1) + (x) = 0
2 + x = 0
x = -2
Therefore, the oxidation number of oxygen in H₂O is -2. This aligns perfectly with the general rule (rule 4) that oxygen typically has an oxidation number of -2, except in specific circumstances.
Exceptions to the Rule: Peroxides and Fluorine Compounds
While -2 is the most common oxidation number for oxygen, it's crucial to remember the exceptions mentioned in rule 4.
Peroxides:
Peroxides contain an oxygen-oxygen single bond (O-O). In these compounds, each oxygen atom has an oxidation number of -1. A classic example is hydrogen peroxide (H₂O₂). Let's verify:
- Each hydrogen atom has an oxidation number of +1 (total +2).
- Let 'x' be the oxidation number of each oxygen atom. There are two oxygen atoms.
- The sum of oxidation numbers must be zero: (+2) + 2x = 0 => x = -1
Thus, the oxidation number of oxygen in H₂O₂ is -1.
Compounds with Fluorine:
Fluorine, being the most electronegative element, can force oxygen to have a positive oxidation number. In compounds like oxygen difluoride (OF₂), oxygen has a +2 oxidation number because fluorine's electronegativity dictates it takes on the negative oxidation number.
Importance of Oxidation Numbers in Redox Reactions
Understanding oxidation numbers is crucial for comprehending redox reactions. Oxidation involves an increase in oxidation number (loss of electrons), while reduction involves a decrease in oxidation number (gain of electrons). In redox reactions, one substance is oxidized while another is reduced. The changes in oxidation numbers allow us to balance redox equations and track electron transfer.
For instance, consider the reaction between hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
In this reaction:
- Hydrogen's oxidation number changes from 0 (in H₂) to +1 (in H₂O). Hydrogen is oxidized.
- Oxygen's oxidation number changes from 0 (in O₂) to -2 (in H₂O). Oxygen is reduced.
The balanced equation reflects the equal number of electrons lost by hydrogen and gained by oxygen.
Applications of Oxidation Numbers
The concept of oxidation numbers extends far beyond simply understanding water's composition. It's fundamental to various applications in chemistry and related fields:
-
Balancing redox equations: Determining oxidation numbers helps balance complex redox reactions accurately.
-
Predicting reaction spontaneity: Oxidation numbers can indicate the potential for a redox reaction to occur spontaneously.
-
Electrochemistry: Oxidation numbers are essential in understanding electrochemical processes, including batteries and corrosion.
-
Analytical chemistry: Oxidation states are utilized in various analytical techniques to identify and quantify elements in compounds.
-
Geochemistry and mineralogy: Oxidation states play a critical role in understanding mineral formation and transformation processes within the Earth.
Beyond H₂O: Oxidation Numbers in Other Compounds
While we've focused on H₂O, the principles discussed apply broadly. Let's consider a few examples:
-
Carbon dioxide (CO₂): Carbon's oxidation number is +4, and each oxygen's oxidation number is -2.
-
Sulfuric acid (H₂SO₄): Hydrogen is +1, oxygen is -2, and sulfur is +6.
-
Potassium permanganate (KMnO₄): Potassium is +1, oxygen is -2, and manganese is +7.
Mastering the concept of oxidation numbers requires practice. Working through various examples, applying the rules, and checking your work will solidify your understanding.
Conclusion: The Significance of Oxidation Number in H₂O and Beyond
The seemingly simple question of oxygen's oxidation number in H₂O opens a gateway to understanding fundamental chemical principles. This article provided a comprehensive exploration of oxidation numbers, their determination, and their crucial role in understanding chemical reactions, particularly redox reactions. The examples provided demonstrate the wide-ranging applications of this concept beyond the confines of a single molecule. By grasping these principles, you’ll be well-equipped to tackle more complex chemical concepts and scenarios. The ability to assign and interpret oxidation numbers is a cornerstone of advanced chemical understanding. Continue practicing, explore diverse examples, and your understanding will grow exponentially.
Latest Posts
Latest Posts
-
Positive And Negative Effects Of Imperialism
Mar 25, 2025
-
How Many Valence Electrons In Cu
Mar 25, 2025
-
What Is The Rhyme Scheme Of These Lines
Mar 25, 2025
-
The Tongue Is Made Of Smooth Muscle
Mar 25, 2025
-
The Two Purines Bases In Dna Are
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of Oxygen In H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
