How Many Valence Electrons In Cu
News Leon
Mar 25, 2025 · 6 min read
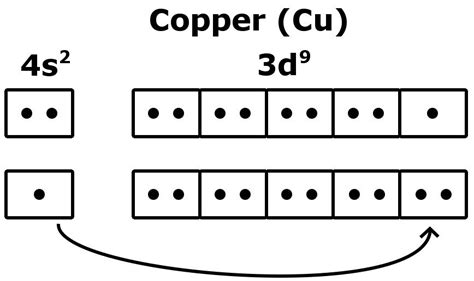
Table of Contents
How Many Valence Electrons Does Copper (Cu) Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Copper (Cu), a reddish-orange metal known for its excellent conductivity and malleability, plays a crucial role in various industries and biological processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its unique chemical and physical properties. This article delves deep into the electronic configuration of copper, explaining why determining its valence electrons isn't as straightforward as it might initially seem, and exploring the implications of this seemingly simple number on its reactivity and applications.
Understanding Valence Electrons: The Foundation of Chemical Bonding
Before focusing specifically on copper, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they participate in chemical bonding, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons directly influences an element's oxidation state and its overall chemical behavior.
For most elements, the number of valence electrons can be readily determined by looking at their group number on the periodic table. However, transition metals, including copper, exhibit slightly more complex behavior.
The Electronic Configuration of Copper: A Twist in the Tale
Copper's atomic number is 29, meaning it has 29 electrons. Based on standard filling rules (Aufbau principle), you might expect its electronic configuration to be: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁹. This configuration suggests that copper would have one valence electron (the 4s electron).
However, this is not the experimentally observed electronic configuration. The actual electronic configuration of copper is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This seemingly small change has significant consequences.
Why the Anomaly? The Stability of a Full d-Subshell
The deviation from the expected configuration arises due to the exceptional stability associated with a completely filled d-subshell. While the 4s subshell is slightly lower in energy than the 3d subshell, the energy difference is minimal. The extra stability gained by having a fully filled 3d subshell (10 electrons) outweighs the slight energy penalty of having only one electron in the 4s subshell. This phenomenon is also observed in other transition metals, albeit less frequently.
This rearrangement effectively means that copper has a single valence electron, even though the 3d electrons can participate in bonding under certain circumstances.
The Implications of Copper's Single Valence Electron
The seemingly simple fact that copper possesses only one valence electron profoundly influences its properties and behavior:
1. Oxidation States: The Versatility of Copper
Copper's single valence electron allows it to readily lose this electron, forming the +1 oxidation state (Cu⁺). However, due to the involvement of the 3d electrons, copper can also exhibit a +2 oxidation state (Cu²⁺), and even less common higher oxidation states under specific conditions. The ability to exist in multiple oxidation states is a characteristic feature of transition metals, and it significantly broadens the range of compounds and reactions that copper can participate in.
The +1 and +2 oxidation states lead to distinct chemical properties and color differences in copper compounds. For example, Cu⁺ compounds often exhibit colors ranging from colorless to white, while Cu²⁺ compounds are often blue or green.
2. Conductivity: A Sea of Mobile Electrons
Copper's excellent electrical and thermal conductivity is directly linked to its electronic configuration. The single valence electron, along with the mobile 3d electrons, contributes to a "sea" of delocalized electrons that can easily move throughout the metal lattice. This free movement of electrons allows for efficient charge and heat transfer, making copper an ideal material for electrical wiring and heat exchangers.
3. Malleability and Ductility: The Advantage of Metallic Bonding
Copper's malleability (ability to be hammered into sheets) and ductility (ability to be drawn into wires) stem from the metallic bonding present in its structure. The delocalized valence electrons act as a "glue," holding the copper atoms together in a strong yet flexible structure. The relatively weak interaction between the positively charged copper ions and the delocalized electrons allows the metal lattice to deform under stress without breaking.
4. Catalytic Activity: A Player in Chemical Reactions
The ability of copper to exist in multiple oxidation states enables it to act as a catalyst in various chemical reactions. Copper catalysts are employed in numerous industrial processes, including the synthesis of organic compounds, the production of ammonia, and oxidation reactions. The ability of copper to readily accept and donate electrons during these reactions is crucial for its catalytic function.
5. Biological Significance: Essential for Life
Copper is an essential trace element for humans and other living organisms. It serves as a cofactor in various enzymes, playing a crucial role in vital processes like respiration, iron metabolism, and connective tissue formation. The ability of copper to exist in multiple oxidation states allows it to participate in redox reactions that are essential for these enzymatic functions.
Beyond the Basics: Delving Deeper into Copper Chemistry
The seemingly simple answer to "how many valence electrons does copper have?"—one—opens the door to a complex and fascinating area of chemistry. The involvement of the 3d electrons and the existence of multiple oxidation states lead to a wide range of copper compounds with diverse properties and applications.
Copper(I) Compounds: The +1 Oxidation State
Copper(I) compounds typically involve Cu⁺ ions, where the single valence electron has been lost. These compounds often exhibit distinct properties due to the filled d¹⁰ configuration, offering unique characteristics that are exploited in various applications. For example, copper(I) oxide (Cu₂O) is a semiconductor material, while copper(I) chloride (CuCl) is used in pyrotechnics and catalysis.
Copper(II) Compounds: The +2 Oxidation State
Copper(II) compounds, featuring Cu²⁺ ions, are significantly more common and exhibit a broader range of colors and properties compared to their copper(I) counterparts. The presence of an unpaired electron in the 3d orbitals leads to paramagnetism, meaning they are attracted to magnetic fields. This property, along with their vivid colors, is useful in many applications such as pigments, catalysts, and coordination complexes. Examples include copper(II) sulfate (CuSO₄), a common fungicide and agricultural chemical, and copper(II) chloride (CuCl₂), used in various catalytic processes.
Advanced Applications: Leveraging Copper's Unique Properties
The unique properties of copper arising from its electronic configuration are exploited in numerous advanced technological applications:
- Electronics: Copper's high electrical conductivity makes it an indispensable material in electronic circuits and wiring.
- Catalysis: Copper catalysts are employed in a wide range of industrial processes, including petroleum refining, ammonia synthesis, and the production of fine chemicals.
- Materials Science: Copper alloys are used in various structural and functional applications due to their strength, corrosion resistance, and other desirable properties.
- Medicine: Copper compounds find applications in medicine as antimicrobial agents, anticancer drugs, and diagnostic tools.
Conclusion: A Single Valence Electron with a Big Impact
While the seemingly simple answer to the question—one valence electron—might appear straightforward, it underpins the remarkable versatility and widespread utility of copper. The unique electronic configuration of copper, specifically the interplay between the 4s and 3d electrons, allows for multiple oxidation states, excellent conductivity, and a range of chemical and physical properties that are crucial for its diverse applications in various fields from electronics and materials science to biology and medicine. Understanding the underlying electronic structure is essential for appreciating the significant impact of this reddish-orange metal on our lives.
Latest Posts
Latest Posts
-
A Man Was Murdered In His Office Riddle
Mar 28, 2025
-
A Coil Is Formed By Winding 250 Turns
Mar 28, 2025
-
A Rotating Fan Completes 1200 Revolutions
Mar 28, 2025
-
1 Meter Equals How Many Millimeters
Mar 28, 2025
-
In The System Of Mass Production Unskilled Workers
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Cu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
