Number Of Valence Electrons In Copper
News Leon
Mar 22, 2025 · 5 min read
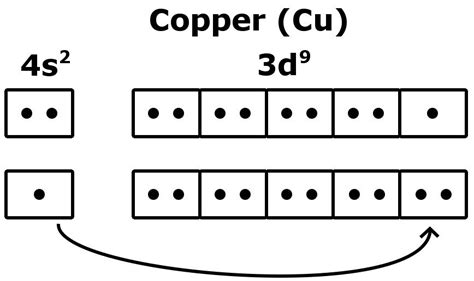
Table of Contents
Unveiling the Mystery: The Number of Valence Electrons in Copper
Copper, a reddish-orange metal renowned for its excellent electrical conductivity and malleability, holds a unique position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to grasping its remarkable properties and diverse applications. This comprehensive article delves deep into the intricacies of copper's valence electrons, exploring its electronic configuration, oxidation states, and how these factors influence its behavior in various chemical and physical contexts.
Copper's Electronic Configuration: The Foundation of Valence
To understand the number of valence electrons in copper, we must first examine its electronic configuration. Copper (Cu) has an atomic number of 29, meaning it possesses 29 electrons. According to the Aufbau principle and Hund's rule, these electrons fill the atomic orbitals in a specific order, resulting in the following electronic configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰
This configuration might seem counterintuitive at first glance. One might expect a 4s² 3d⁹ configuration. However, a filled d subshell (d¹⁰) is more stable than a partially filled one (d⁹). This subtle but significant difference accounts for many of copper's unique properties.
The Anomaly and Stability: Why 4s¹ 3d¹⁰?
The seemingly anomalous 4s¹ 3d¹⁰ configuration arises from the relatively small energy difference between the 4s and 3d orbitals. Filling the 3d subshell completely results in a state of higher stability, overriding the Aufbau principle's prediction. This enhanced stability is a direct consequence of the electron-electron interactions within the atom. A fully filled d-orbital minimizes electron-electron repulsion, leading to a lower overall energy state.
Defining Valence Electrons: The Outermost Players
Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. They are the key players determining an element's reactivity and the types of chemical bonds it can form. In most cases, valence electrons are located in the highest principal energy level (n).
Identifying Valence Electrons in Copper: A Deeper Dive
While the simplistic view might suggest only one valence electron (the 4s¹ electron), the situation with copper is more nuanced. Although the 3d electrons are not as readily involved in bonding as the 4s electron, they can participate in chemical reactions, particularly in the formation of complexes and compounds exhibiting higher oxidation states. Therefore, we must consider both the 4s and 3d electrons when fully characterizing copper's valency.
Copper's Oxidation States: A Manifestation of Valence Electrons
The oxidation state of an element indicates the number of electrons it has gained or lost in a chemical reaction. Copper exhibits variable oxidation states, primarily +1 (cuprous) and +2 (cupric), showcasing the involvement of its valence electrons in bonding.
+1 Oxidation State (Cuprous): A Single Electron's Journey
In the +1 oxidation state, copper loses its single 4s electron. This results in a configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰. Compounds like copper(I) oxide (Cu₂O) and copper(I) chloride (CuCl) exemplify this oxidation state.
+2 Oxidation State (Cupric): A More Involved Participation
The +2 oxidation state involves the loss of both the 4s electron and one 3d electron, resulting in a configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁹. This oxidation state is significantly more common, seen in compounds such as copper(II) sulfate (CuSO₄) and copper(II) oxide (CuO). The involvement of a 3d electron illustrates the contribution of these electrons to copper's chemical reactivity.
Higher Oxidation States: Rare but Possible
While less common, copper can also exhibit higher oxidation states, such as +3. These states are usually observed in specific chemical environments and complex compounds. In these instances, more than one 3d electron is involved in the bonding process.
The Influence of Valence Electrons on Copper's Properties
The number and behavior of copper's valence electrons directly affect its remarkable properties:
Electrical Conductivity: A Sea of Mobile Electrons
Copper's excellent electrical conductivity stems from its loosely held valence electrons. These electrons are relatively mobile and can easily move through the copper lattice, facilitating the flow of electrical current. The 4s and even some 3d electrons contribute to this sea of mobile charge carriers.
Thermal Conductivity: Efficient Heat Transfer
Similar to its electrical conductivity, copper's high thermal conductivity is a consequence of the mobility of its valence electrons. These electrons efficiently transfer kinetic energy throughout the material, resulting in rapid heat propagation.
Malleability and Ductility: Adaptable Atomic Structure
Copper's malleability (ability to be shaped) and ductility (ability to be drawn into wires) are attributed to its metallic bonding and the arrangement of its atoms in a face-centered cubic structure. The relatively weak interactions between the copper atoms allow them to slide past each other under stress without fracturing the material. The valence electrons contribute significantly to the metallic bonding, facilitating this atomic rearrangement.
Applications Leveraging Copper's Valence Electrons
The properties stemming from copper's unique valence electron arrangement have led to its widespread use in countless applications:
-
Electrical Wiring: Copper's high conductivity makes it the material of choice for electrical wiring in homes, buildings, and power grids.
-
Electronics: Its conductivity and ability to be easily processed into thin films make copper crucial in microelectronics and printed circuit boards.
-
Plumbing: Copper pipes are used extensively in plumbing systems due to their resistance to corrosion and durability.
-
Coins and Alloys: Copper forms many important alloys such as brass and bronze, which possess enhanced strength and other desirable properties.
Conclusion: A Comprehensive Understanding of Copper's Valence Electrons
In conclusion, while a simplistic approach might suggest one valence electron for copper, a thorough investigation reveals a more complex picture. The interplay between the 4s and 3d electrons, and their participation in chemical bonding, fully describes copper's behavior and its diverse applications. Understanding the number of valence electrons in copper, their influence on its electronic configuration and oxidation states, and their contribution to its unique physical and chemical properties is crucial for anyone working with this versatile and essential metal. The subtle nuances in its electronic structure highlight the complexity and elegance of atomic-level interactions and their macroscopic consequences. Further research continues to explore the finer points of copper's electronic structure and its implications for material science and technology.
Latest Posts
Latest Posts
-
What Is Meant By Regional Political Party
Mar 24, 2025
-
How To Find Boiling Point Of A Solution
Mar 24, 2025
-
What Is The Molar Mass Of Nh4 2co3
Mar 24, 2025
-
Which Of The Following Are Included In Compensation Of Employees
Mar 24, 2025
-
Difference Between A Hormone And An Enzyme
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Copper . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
