Number Of Electrons In A 3s Sublevel
News Leon
Mar 15, 2025 · 6 min read
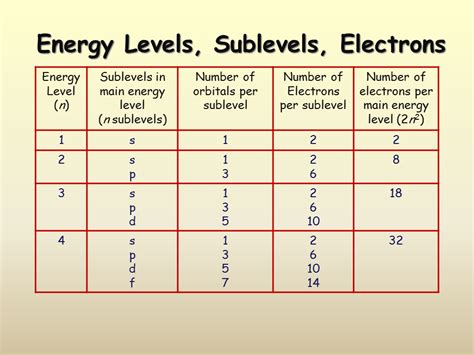
Table of Contents
Number of Electrons in a 3s Sublevel: A Deep Dive into Atomic Structure
Understanding the number of electrons in a 3s sublevel is fundamental to grasping atomic structure and the behavior of elements. This article delves deep into this concept, explaining the underlying principles of electron configuration, quantum numbers, and the implications for chemical properties. We'll explore this topic comprehensively, ensuring a thorough understanding for both beginners and those seeking a more advanced perspective.
Understanding Electron Shells, Subshells, and Orbitals
Before we dive into the specifics of the 3s sublevel, let's establish a solid foundation in atomic structure. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons occupying various energy levels.
Electron Shells (Principal Energy Levels):
Electrons reside in shells, representing their principal energy levels. These shells are designated by the principal quantum number (n), where n = 1, 2, 3, and so on, representing increasing energy levels. The further an electron is from the nucleus (higher n value), the higher its energy.
Electron Subshells (Azimuthal Quantum Number):
Within each shell, electrons are further organized into subshells. These subshells are characterized by the azimuthal quantum number (l), which can have values from 0 to n-1. Each value of 'l' corresponds to a specific subshell:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Electron Orbitals (Magnetic Quantum Number):
Each subshell contains one or more orbitals. Orbitals are regions of space where there's a high probability of finding an electron. The number of orbitals in a subshell is determined by the magnetic quantum number (ml), which can have values from -l to +l, including 0.
- s subshell (l=0): Contains 1 orbital.
- p subshell (l=1): Contains 3 orbitals (ml = -1, 0, +1).
- d subshell (l=2): Contains 5 orbitals (ml = -2, -1, 0, +1, +2).
- f subshell (l=3): Contains 7 orbitals (ml = -3, -2, -1, 0, +1, +2, +3).
The 3s Sublevel: Location and Electron Capacity
Now, let's focus on the 3s sublevel. The '3' indicates that it's in the third principal energy level (shell), and the 's' signifies that it's an s subshell. As we established earlier, an s subshell always contains only one orbital.
Crucially, each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle. This principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms, where ms is the spin quantum number, representing the electron's spin: +1/2 or -1/2).
Therefore, since the 3s sublevel has one orbital, it can hold a maximum of two electrons.
Electron Configuration and the 3s Sublevel
Electron configuration describes the arrangement of electrons in an atom's shells and subshells. It follows a specific filling order, generally determined by the Aufbau principle (filling lower energy levels first) and Hund's rule (maximizing unpaired electrons in a subshell before pairing them).
For example, let's consider the element sodium (Na), which has 11 electrons. Its electron configuration is 1s²2s²2p⁶3s¹. Notice the single electron in the 3s sublevel. Magnesium (Mg), with 12 electrons, has the configuration 1s²2s²2p⁶3s². In this case, the 3s sublevel is completely filled with its two electrons.
Implications of the 3s Sublevel's Electron Occupancy
The number of electrons in the 3s sublevel significantly influences an element's chemical properties. Elements with a partially filled 3s sublevel are typically more reactive than those with a completely filled 3s sublevel. This is because elements strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons to fill their outermost shell (valence shell).
For example, sodium readily loses its single 3s electron to achieve the stable electron configuration of neon (1s²2s²2p⁶), forming a +1 ion. Magnesium, with two 3s electrons, can lose both to achieve the same stable configuration, forming a +2 ion. This reactivity is crucial in understanding chemical bonding and the formation of compounds.
Advanced Concepts and Exceptions
While the Aufbau principle generally predicts electron configuration, there are exceptions. These exceptions often involve the subtle energy differences between subshells, particularly in transition metals and lanthanides/actinides. These energy differences can lead to slight variations in the expected electron configuration.
The Role of Quantum Numbers in Defining the 3s Sublevel
Let's revisit the quantum numbers and how they define the 3s sublevel:
-
Principal Quantum Number (n = 3): This indicates the third energy level, further from the nucleus than the 1s and 2s orbitals. This implies higher energy for the electrons within the 3s sublevel.
-
Azimuthal Quantum Number (l = 0): This designates the s subshell, characterized by a spherical orbital shape. The spherical symmetry means the electron probability density is distributed uniformly around the nucleus.
-
Magnetic Quantum Number (ml = 0): Since l = 0, ml can only be 0. This means there's only one orbital within the 3s subshell, able to hold a maximum of two electrons with opposite spins.
-
Spin Quantum Number (ms = +1/2 or -1/2): This specifies the intrinsic angular momentum (spin) of the electron. Each orbital in the 3s subshell can hold one electron with spin +1/2 and one electron with spin -1/2.
Relating the 3s Sublevel to Periodic Trends
The filling of the 3s sublevel directly impacts periodic trends:
-
Atomic Radius: As you move down a group (column) in the periodic table, the principal quantum number (n) increases, leading to larger atomic radii. Elements with electrons in the 3s sublevel generally have larger atomic radii than those with electrons in the 2s sublevel.
-
Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. The ionization energy for removing a 3s electron is lower than that for removing a 2s electron because the 3s electron is further from the nucleus and experiences less effective nuclear charge.
-
Electronegativity: Electronegativity is the ability of an atom to attract electrons in a chemical bond. Electronegativity generally decreases as you move down a group, so elements with 3s electrons tend to have lower electronegativity than those with 2s electrons.
Practical Applications and Significance
The understanding of the 3s sublevel and electron configuration is not just theoretical; it has significant practical applications:
-
Chemistry: Predicting chemical reactivity, bonding behavior, and the formation of compounds.
-
Material Science: Designing new materials with specific electrical, magnetic, or optical properties. The electron configuration, including the occupancy of the 3s sublevel, influences these properties.
-
Spectroscopy: Analyzing the absorption and emission of light by atoms, which is directly related to electron transitions between energy levels, including transitions involving the 3s sublevel.
Conclusion
The seemingly simple question of the number of electrons in a 3s sublevel opens a gateway to a deep understanding of atomic structure, quantum mechanics, and the periodic properties of elements. The ability to predict and explain the behavior of atoms based on their electron configurations, particularly the occupancy of subshells like the 3s, is fundamental to various scientific disciplines. By comprehending the principles discussed here, we can gain a more profound appreciation for the intricate world of atomic-level interactions and their influence on the macroscopic properties of matter.
Latest Posts
Latest Posts
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
-
Mountain Range That Separates Europe And Asia
Mar 15, 2025
-
16 Out Of 40 As A Percentage
Mar 15, 2025
-
Which Of The Following Is A True Solution
Mar 15, 2025
-
How Many Vertices Does A Rectangular Pyramid Have
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Number Of Electrons In A 3s Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
