Net Ionic Equation For Hcl + Naoh
News Leon
Mar 26, 2025 · 6 min read
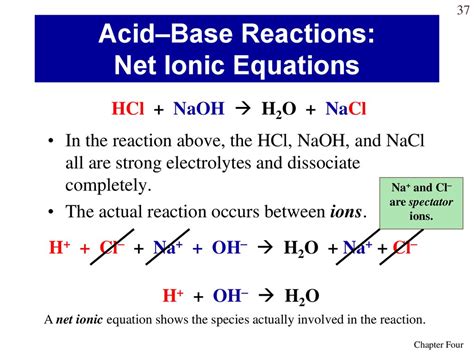
Table of Contents
Net Ionic Equation for HCl + NaOH: A Deep Dive into Acid-Base Reactions
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, particularly its net ionic equation, is fundamental to grasping the concepts of acid-base chemistry and stoichiometry. This article provides a comprehensive exploration of this reaction, explaining the process of deriving the net ionic equation, discussing its implications, and exploring related concepts.
Understanding the Molecular Equation
Before diving into the net ionic equation, let's first examine the molecular equation for the reaction between HCl and NaOH:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation shows the reactants, HCl (hydrochloric acid) and NaOH (sodium hydroxide), reacting to form the products, NaCl (sodium chloride) and H₂O (water). The "(aq)" designation indicates that the substance is dissolved in water (aqueous solution), while "(l)" indicates it's in liquid form.
This molecular equation accurately represents the overall reaction, but it doesn't reveal the actual ions involved. To gain a deeper understanding, we need to break down the equation into its ionic components.
Dissecting the Complete Ionic Equation
Strong acids and strong bases dissociate completely in aqueous solutions. This means they break apart into their constituent ions. HCl and NaOH are both strong electrolytes, meaning they fully ionize in water:
- HCl(aq) → H⁺(aq) + Cl⁻(aq)
- NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Substituting these dissociated ions into the molecular equation gives us the complete ionic equation:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation shows all the ions present in the solution before and after the reaction. Notice that some ions appear on both sides of the equation.
Deriving the Net Ionic Equation: The Essence of the Reaction
The net ionic equation focuses solely on the species that actually participate in the chemical change. Ions that appear on both the reactant and product sides are spectator ions. They don't undergo any transformation during the reaction. In this case, Na⁺(aq) and Cl⁻(aq) are spectator ions. They are present in solution but don't actively participate in the reaction.
To obtain the net ionic equation, we eliminate the spectator ions from the complete ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This is the net ionic equation for the reaction between HCl and NaOH. It concisely represents the fundamental chemical change: the reaction between hydrogen ions (H⁺) and hydroxide ions (OH⁻) to form water (H₂O). This equation is independent of the specific strong acid and strong base used; it represents the essence of all strong acid-strong base neutralization reactions.
Significance of the Net Ionic Equation
The net ionic equation provides several crucial insights:
- Simplicity and Clarity: It simplifies the representation of the reaction, focusing on the essential chemical transformation.
- General Applicability: It applies to all neutralization reactions involving strong acids and strong bases, regardless of the specific acid and base used. The underlying chemical process remains the same.
- Stoichiometric Ratios: It reveals the stoichiometric ratio between the reacting ions. In this case, one mole of H⁺ reacts with one mole of OH⁻ to produce one mole of H₂O.
- Predicting Products: It helps predict the products of similar reactions involving strong acids and strong bases.
- Understanding Solution Chemistry: It helps in understanding the behavior of ions in aqueous solutions and the concept of spectator ions.
Exploring Variations and Applications
While the HCl + NaOH reaction is a straightforward example, variations exist. Understanding these variations strengthens the fundamental principles:
-
Different Strong Acids and Bases: The net ionic equation remains the same even if different strong acids (like HNO₃, HBr, HI) or strong bases (like KOH, Ca(OH)₂ – note that the stoichiometry changes with polyprotic bases) are used. The essential reaction is always the combination of H⁺ and OH⁻ to form water.
-
Titration Applications: The reaction is fundamental to acid-base titrations, where the known concentration of one reactant (e.g., NaOH) is used to determine the unknown concentration of the other (e.g., HCl). The stoichiometry derived from the net ionic equation is crucial for these calculations.
-
pH Changes: The reaction leads to a significant change in pH. The initial solution is highly basic (NaOH solution), while the final solution after complete neutralization is neutral (pH 7), assuming equal amounts of acid and base are used. Understanding this pH change is vital in various applications, including buffer solutions and pH control.
Beyond the Basics: Weak Acids and Bases
The concept of net ionic equations also applies to reactions involving weak acids and weak bases, although the process is slightly more complex. Weak acids and bases don't fully dissociate in water. Therefore, the complete ionic equation and subsequent net ionic equation will include undissociated molecules of the weak acid or base. For example, the reaction between acetic acid (CH₃COOH) and NaOH would not simplify to just H⁺ + OH⁻ → H₂O. The equilibrium of acetic acid dissociation needs to be considered.
Practical Applications and Real-World Examples
The neutralization reaction between HCl and NaOH has wide-ranging applications in various fields:
- Industrial Processes: Neutralization is used to adjust the pH of industrial waste streams before disposal. Maintaining the correct pH is crucial for environmental protection.
- Chemical Synthesis: Controlled neutralization reactions are employed in many chemical synthesis processes to create specific products or maintain the desired reaction conditions.
- Food and Beverage Industry: pH control is essential in food and beverage production. Neutralization reactions help maintain the desired pH range for optimal product quality and safety.
- Medicine and Pharmaceuticals: Acid-base reactions are crucial in pharmaceutical formulations and drug delivery systems. Understanding neutralization reactions is essential for designing and controlling drug effectiveness and stability.
Advanced Concepts: Enthalpy and Entropy
The HCl + NaOH reaction is exothermic, meaning it releases heat. This heat release is due to the formation of strong bonds in water molecules. The enthalpy change (ΔH) associated with this reaction is negative, indicating an exothermic process. The entropy change (ΔS) is also involved, relating to the change in disorder or randomness of the system. The reaction involves the formation of a more ordered system (water molecules), hence the entropy change might be negative, but the overall Gibbs Free Energy change (ΔG) is negative indicating a spontaneous reaction.
Conclusion: A Foundation in Chemistry
The net ionic equation for the reaction between HCl and NaOH, H⁺(aq) + OH⁻(aq) → H₂O(l), provides a simplified yet powerful representation of a fundamental chemical process. Understanding this equation and its derivation is crucial for mastering concepts in acid-base chemistry, stoichiometry, and solution chemistry. Its applications extend far beyond the classroom, underpinning many industrial processes, scientific investigations, and everyday applications. By delving into the nuances of this seemingly simple reaction, we gain a deeper appreciation for the complexities and elegance of chemical interactions. The implications are vast, ranging from environmental protection to the advancement of medicine and material science. This deep understanding of this simple equation lays the groundwork for exploring more complex chemical systems and reaction mechanisms.
Latest Posts
Latest Posts
-
What Is 0 025 As A Percentage
Mar 29, 2025
-
Integrated Rate Law For Zero Order Reaction
Mar 29, 2025
-
Boiling Water Chemical Or Physical Change
Mar 29, 2025
-
The First Step In Urine Formation Is
Mar 29, 2025
-
What Is The End Product Of Fat Digestion
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation For Hcl + Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
