Boiling Water Chemical Or Physical Change
News Leon
Mar 29, 2025 · 6 min read
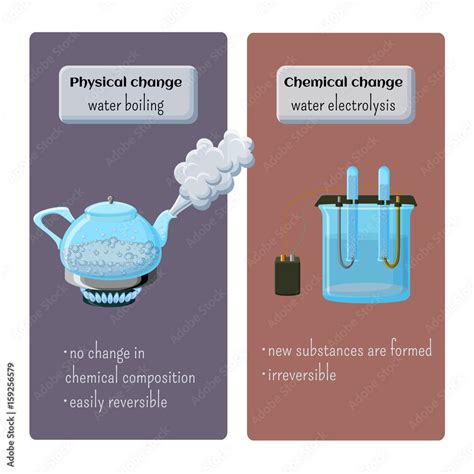
Table of Contents
Boiling Water: A Physical Change Explained
Is boiling water a chemical or physical change? This seemingly simple question leads to a deeper understanding of matter and its transformations. The answer, unequivocally, is that boiling water is a physical change. This article will delve into the reasons why, exploring the scientific principles involved and dispelling common misconceptions. We'll also discuss the differences between physical and chemical changes, examine the properties of water in its different states, and explore related concepts like evaporation and condensation. By the end, you'll have a comprehensive grasp of this fundamental scientific concept.
Understanding Physical and Chemical Changes
Before we delve into the specifics of boiling water, let's establish a clear understanding of the distinction between physical and chemical changes. This foundational knowledge is crucial for grasping the essence of the boiling process.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. The fundamental building blocks—the molecules—remain the same. Think of cutting paper, melting ice, or dissolving sugar in water. In each instance, the substance changes form, but its chemical identity remains unchanged. You could, in theory, reverse these changes and recover the original substance.
Chemical Changes: Altering the Molecular Structure
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms to form new molecules. This results in a substance with different chemical properties than the original. Burning wood, rusting iron, or cooking an egg are all examples of chemical changes. The original substances are transformed into entirely new substances with different characteristics. These changes are typically irreversible.
The Boiling Process: A Detailed Look
Now, let's focus our attention on the process of boiling water. When you heat water, you're essentially increasing the kinetic energy of its molecules. These molecules are in constant motion, and as their energy increases, their movement becomes more vigorous.
From Liquid to Gas: The Phase Transition
At a specific temperature—100° Celsius (212° Fahrenheit) at standard atmospheric pressure—the water molecules gain enough kinetic energy to overcome the intermolecular forces holding them together in the liquid state. This is the boiling point. At this point, the water begins to rapidly transform from a liquid into a gas, a process known as vaporization or boiling.
The Role of Heat Energy
The added heat energy doesn't change the chemical makeup of the water molecules (H₂O). It simply provides the energy needed for the molecules to transition from a more ordered, closely packed liquid state to a more disordered, widely dispersed gaseous state. The water molecules remain H₂O throughout the boiling process.
Key Characteristics of Boiling:
- Rapid Vaporization: Boiling is characterized by rapid vaporization, forming visible bubbles throughout the liquid. This contrasts with evaporation, which occurs more slowly at the surface of the liquid.
- Constant Temperature: During the boiling process, the temperature of the water remains constant at its boiling point until all the water has vaporized. Adding more heat simply increases the rate of vaporization, not the temperature of the water.
- Reversibility: The gaseous water (steam) can be condensed back into liquid water by cooling it. This demonstrates the physical nature of the change.
Differentiating Boiling from Other Processes
It's important to distinguish boiling from other processes that might seem similar, but are fundamentally different.
Evaporation vs. Boiling: A Subtle Difference
Evaporation is a slower process of vaporization that occurs at the surface of a liquid at temperatures below its boiling point. It's driven by the kinetic energy of the most energetic molecules escaping the liquid's surface. Boiling, on the other hand, is a more rapid and vigorous process involving vaporization throughout the entire liquid.
Decomposition: A Chemical Change
Decomposition is a chemical change involving the breakdown of a compound into simpler substances. While heating water might seem like decomposition, it's not. Water molecules (H₂O) remain intact during boiling; they simply change their state of matter. True decomposition of water requires a significant input of energy and would produce hydrogen and oxygen gases, which is a chemical change.
Water's Properties Across Different States
Understanding the properties of water in its different states—solid (ice), liquid (water), and gas (steam)—reinforces the idea that boiling is a physical change.
Solid (Ice): Ordered Structure
In its solid state, water molecules are arranged in a highly ordered crystalline structure. The intermolecular forces are strong enough to hold the molecules in fixed positions.
Liquid (Water): Less Ordered Structure
In its liquid state, water molecules are still relatively close together, but their arrangement is less ordered. They can move around more freely, resulting in the fluidity of liquid water.
Gas (Steam): Highly Disordered Structure
In its gaseous state (steam), water molecules are widely dispersed, moving independently with high kinetic energy. The intermolecular forces are weak, allowing for significant expansion.
The Importance of Understanding Phase Transitions
The phase transitions of water, including boiling, are fundamental to many natural processes and technological applications. Understanding these processes is crucial in various fields:
- Meteorology: The water cycle, involving evaporation, condensation, and precipitation, is essential for weather patterns.
- Chemistry: Phase transitions are crucial for understanding chemical reactions and separations.
- Engineering: Boiling and other phase transitions are critical in many industrial processes, such as power generation and refrigeration.
- Biology: Water's unique properties are fundamental to life, and its phase transitions play a crucial role in biological processes.
Dispelling Common Misconceptions
Several misconceptions surround the boiling of water. Let's address some of the most prevalent ones:
Misconception 1: Boiling water is chemically altered.
Reality: The chemical composition of water (H₂O) remains unchanged during boiling. Only the physical state changes.
Misconception 2: Boiling purifies water.
Reality: Boiling can kill some microorganisms, but it doesn't remove dissolved minerals or chemicals. To obtain purified water, other methods like distillation or filtration are necessary.
Misconception 3: The bubbles in boiling water are oxygen or hydrogen.
Reality: The bubbles in boiling water are primarily water vapor (steam). The release of dissolved gases might occur as well, but the majority of the bubbles consist of gaseous water.
Conclusion: Boiling Water – A Physical Transformation
In conclusion, boiling water is a prime example of a physical change. The process involves a phase transition from liquid to gas, driven by the increased kinetic energy of water molecules. The chemical composition of the water remains unchanged throughout the boiling process. Understanding this distinction between physical and chemical changes is fundamental to understanding the behavior of matter and its transformations. The properties of water in its different states, the differences between boiling and evaporation, and the ability to reverse the process through condensation all solidify the conclusion that boiling water is a purely physical change. This knowledge is not only crucial for understanding basic scientific principles but also has significant implications across various scientific and technological disciplines.
Latest Posts
Latest Posts
-
Each Hemoglobin Molecule Can Transport Two Molecules Of Oxygen
Apr 01, 2025
-
During Meiosis Crossing Over Takes Place Between
Apr 01, 2025
-
If Supply And Demand Both Decrease
Apr 01, 2025
-
Which Of The Following Best Describes Climate
Apr 01, 2025
-
What Are The Parts Of A Solution
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
