Each Hemoglobin Molecule Can Transport Two Molecules Of Oxygen
News Leon
Apr 01, 2025 · 6 min read
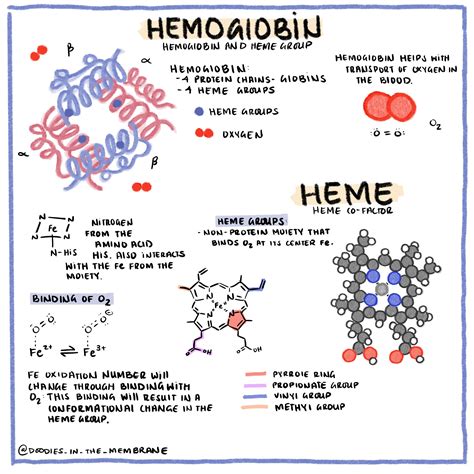
Table of Contents
Debunking the Myth: Each Hemoglobin Molecule Can Transport Four, Not Two, Oxygen Molecules
The statement "each hemoglobin molecule can transport two molecules of oxygen" is incorrect. A single hemoglobin molecule can actually bind and transport four oxygen molecules. This fundamental fact is crucial to understanding how our bodies effectively deliver oxygen to tissues and organs. Let's delve deeper into the structure and function of hemoglobin to dispel this misconception and explore the intricacies of oxygen transport.
Understanding the Structure of Hemoglobin: The Key to Oxygen Binding
Hemoglobin, a metalloprotein found in red blood cells, is the primary oxygen carrier in vertebrates. Its remarkable ability to bind and release oxygen efficiently is directly linked to its unique quaternary structure. Understanding this structure is key to appreciating how it can transport four oxygen molecules.
The Hemoglobin Tetramer: A Complex of Four Subunits
Hemoglobin is not a single, simple protein; it's a tetramer, meaning it's composed of four individual protein subunits. These subunits are arranged in a specific configuration, creating a complex and highly efficient oxygen-binding machine. In adult humans, the hemoglobin tetramer consists of two alpha (α) and two beta (β) globin chains. Each of these globin chains cradles a heme group.
The Heme Group: The Oxygen-Binding Site
The heme group is the heart of the oxygen-binding process. It's a porphyrin ring structure containing a central iron (Fe) ion. This iron ion is the actual site where oxygen binds. Critically, each of the four globin chains in a hemoglobin molecule has its own heme group, resulting in four potential oxygen-binding sites within a single hemoglobin molecule.
Cooperative Binding: The Power of Teamwork
The binding of oxygen to one heme group doesn't occur independently; it influences the binding affinity of the other heme groups. This phenomenon is known as cooperative binding. The binding of the first oxygen molecule to a heme group induces a conformational change in the hemoglobin molecule, making it easier for subsequent oxygen molecules to bind to the remaining heme groups. This cooperative effect significantly increases the efficiency of oxygen uptake in the lungs where oxygen partial pressure is high.
Oxygen Saturation Curve: Visualizing Cooperative Binding
The cooperative binding of oxygen to hemoglobin is beautifully illustrated by the oxygen-hemoglobin dissociation curve, also known as the oxygen saturation curve. This sigmoid-shaped curve demonstrates the gradual increase in hemoglobin saturation with increasing partial pressure of oxygen. The steep portion of the curve in the physiological range highlights the efficiency of oxygen loading in the lungs and unloading in the tissues.
The Process of Oxygen Transport: From Lungs to Tissues
The journey of oxygen, from its inhalation in the lungs to its delivery to the body's tissues, is a fascinating example of biological efficiency, heavily reliant on the four oxygen-binding sites of hemoglobin.
Oxygen Loading in the Lungs: High Partial Pressure
In the lungs, the partial pressure of oxygen (PO2) is high. This high PO2 drives the binding of oxygen to the heme groups within hemoglobin. The cooperative binding ensures that even at relatively low PO2 levels, a substantial amount of oxygen gets loaded onto the hemoglobin molecules. The fully saturated hemoglobin molecule, carrying its full complement of four oxygen molecules, then enters the bloodstream.
Oxygen Unloading in the Tissues: Low Partial Pressure
As the oxygenated blood reaches the tissues, the situation reverses. The partial pressure of oxygen (PO2) in the tissues is significantly lower than in the lungs. This low PO2 promotes the release of oxygen from hemoglobin. Again, the cooperative binding plays a crucial role, making it easier for the already released oxygen molecules to further dissociate from the remaining heme groups.
Factors Affecting Oxygen Release: pH, Temperature, and 2,3-Bisphosphoglycerate (2,3-BPG)
The efficiency of oxygen release from hemoglobin isn't solely dependent on the PO2. Several other factors influence the oxygen-hemoglobin dissociation curve, shifting it to the right (favoring oxygen release) or left (favoring oxygen binding):
-
pH: A decrease in pH (increase in acidity), such as during strenuous exercise, shifts the curve to the right, promoting oxygen release to metabolically active tissues. This is known as the Bohr effect.
-
Temperature: An increase in temperature, also associated with increased metabolic activity, shifts the curve to the right, enhancing oxygen unloading.
-
2,3-Bisphosphoglycerate (2,3-BPG): This molecule binds to hemoglobin, reducing its affinity for oxygen and shifting the curve to the right. Its concentration increases under hypoxic conditions, promoting oxygen release to tissues starved of oxygen.
Clinical Significance: Hemoglobin Disorders and Oxygen Transport
Understanding the function of hemoglobin and its ability to transport four oxygen molecules is crucial for diagnosing and treating various hemoglobin disorders. These disorders often compromise the oxygen-carrying capacity of the blood, leading to significant health consequences.
Anemia: Reduced Oxygen-Carrying Capacity
Anemia, characterized by a deficiency of red blood cells or hemoglobin, directly impacts oxygen transport. Reduced numbers of red blood cells or decreased hemoglobin concentration mean fewer available oxygen-binding sites, resulting in reduced oxygen delivery to the tissues. Various types of anemia exist, with different underlying causes and varying degrees of severity.
Sickle Cell Anemia: Altered Hemoglobin Structure
Sickle cell anemia is a genetic disorder resulting from a mutation in the beta-globin chain of hemoglobin. This mutation alters the structure of hemoglobin, causing it to polymerize under low-oxygen conditions, forming rigid, sickle-shaped red blood cells. These misshapen cells obstruct blood flow, leading to pain crises, organ damage, and other complications. The impaired oxygen transport further exacerbates the condition.
Thalassemia: Impaired Hemoglobin Synthesis
Thalassemia is a group of inherited blood disorders characterized by reduced or absent synthesis of globin chains. This deficiency results in abnormal hemoglobin production and a decreased capacity to carry oxygen. The severity of thalassemia varies depending on the type and extent of globin chain deficiency.
Carbon Monoxide Poisoning: Competitive Inhibition
Carbon monoxide (CO) is a colorless, odorless gas that binds to hemoglobin with much higher affinity than oxygen. This competitive inhibition effectively blocks oxygen binding, leading to hypoxia (oxygen deficiency) and potentially fatal consequences. The high affinity of CO for hemoglobin means that even at low CO concentrations, a significant portion of hemoglobin can be rendered incapable of transporting oxygen.
Conclusion: The Four-Oxygen Capacity is Crucial for Life
The notion that hemoglobin can only transport two oxygen molecules is a significant misunderstanding. The ability of each hemoglobin molecule to bind and transport four oxygen molecules is fundamental to the efficient oxygen delivery system in our bodies. This capacity, coupled with the cooperative binding effect and the influence of factors like pH, temperature, and 2,3-BPG, ensures that our tissues receive the oxygen they need to function optimally. Understanding the intricate mechanisms of oxygen transport is not merely academic; it's crucial for comprehending various clinical conditions that affect oxygen delivery and for developing effective treatments. The four oxygen-binding sites of hemoglobin are a testament to the exquisite design and efficiency of biological systems. The misrepresentation of this vital aspect can lead to a flawed understanding of respiratory physiology and related diseases. Always remember, it’s the four oxygen molecules carried by each hemoglobin that sustain life.
Latest Posts
Latest Posts
-
Which Of The Following Pairs Are Mismatched
Apr 02, 2025
-
Why Displacement Is A Vector Quantity
Apr 02, 2025
-
What Are The Coordinates Of Point S
Apr 02, 2025
-
Essay About The Effects Of Social Media
Apr 02, 2025
-
A Solid Conducting Sphere Of Radius
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Each Hemoglobin Molecule Can Transport Two Molecules Of Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
