Net Ionic Equation For Hcl And Naoh
News Leon
Mar 23, 2025 · 5 min read
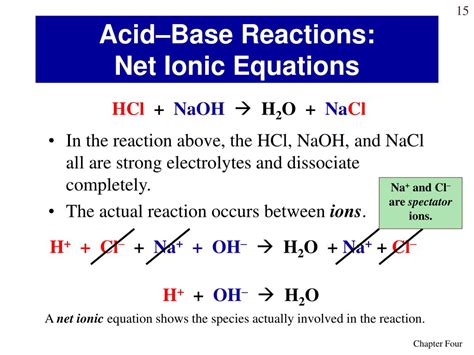
Table of Contents
Net Ionic Equation for HCl and NaOH: A Comprehensive Guide
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction, particularly at the net ionic equation level, is crucial for grasping the underlying principles of acid-base chemistry. This article will delve into the intricacies of this reaction, explaining the balanced molecular equation, the complete ionic equation, and finally, the crucial net ionic equation. We’ll also explore the implications and applications of this seemingly simple reaction.
Understanding the Reaction: HCl + NaOH
Hydrochloric acid (HCl) is a strong acid, meaning it completely dissociates in water into hydrogen ions (H⁺) and chloride ions (Cl⁻). Sodium hydroxide (NaOH), on the other hand, is a strong base, completely dissociating in water into sodium ions (Na⁺) and hydroxide ions (OH⁻). When these two solutions are mixed, a neutralization reaction occurs, producing water (H₂O) and sodium chloride (NaCl), a salt.
The Balanced Molecular Equation
The balanced molecular equation represents the overall reaction, showing the reactants and products as complete molecules:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation tells us that one mole of hydrochloric acid reacts with one mole of sodium hydroxide to produce one mole of sodium chloride and one mole of water. The (aq) indicates that the substance is dissolved in water (aqueous), while (l) indicates it's a liquid.
Delving Deeper: The Complete Ionic Equation
The complete ionic equation provides a more detailed picture of the reaction, showing all the ions present in the solution before and after the reaction. Since HCl and NaOH are strong electrolytes, they completely dissociate into their constituent ions:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation shows that the reaction involves the interaction of hydrogen ions (H⁺) and hydroxide ions (OH⁻) to form water. Sodium ions (Na⁺) and chloride ions (Cl⁻) are present both before and after the reaction, remaining unchanged. These are called spectator ions.
The Essence of the Reaction: The Net Ionic Equation
The net ionic equation simplifies the complete ionic equation by removing the spectator ions. These spectator ions are not directly involved in the chemical change; they simply remain dissolved in the solution. By eliminating them, we obtain the net ionic equation, which represents the core chemical process:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation clearly shows that the essence of the neutralization reaction between HCl and NaOH is the combination of hydrogen ions and hydroxide ions to form water. This is the most important aspect of the reaction. The formation of water is an exothermic process, meaning it releases heat. This heat release is a characteristic feature of acid-base neutralization reactions.
Significance and Applications of the Net Ionic Equation
The net ionic equation is crucial for several reasons:
- Understanding the Reaction Mechanism: It isolates the essential chemical change, providing a clearer understanding of the reaction mechanism at the ionic level.
- Predicting Reactions: Knowing the net ionic equation allows for the prediction of similar reactions between different strong acids and strong bases. The net ionic equation will always be the same for the reaction between any strong acid and strong base.
- Stoichiometric Calculations: It simplifies stoichiometric calculations, focusing only on the reacting species.
- Solubility Predictions: The net ionic equation can help predict the formation of precipitates in reactions involving salts.
Applications in Various Fields
The neutralization reaction between HCl and NaOH, and consequently its net ionic equation, finds applications across various fields:
- Titrations: This reaction is fundamental to acid-base titrations, a widely used analytical technique to determine the concentration of an unknown acid or base. By carefully measuring the volume of NaOH required to neutralize a known volume of HCl, the concentration of HCl can be precisely determined.
- Industrial Processes: Neutralization reactions are used extensively in various industrial processes to control pH levels. For example, wastewater treatment often involves neutralizing acidic or basic effluents to make them environmentally safe.
- Medicine: The neutralization of stomach acid (which contains HCl) is a key principle behind the action of antacids. Many antacids contain bases that neutralize excess stomach acid, relieving heartburn and indigestion.
- Chemical Synthesis: Controlled neutralization reactions are crucial in many chemical syntheses to create specific pH conditions necessary for particular reactions to occur.
Beyond Strong Acids and Strong Bases: A Broader Perspective
While this article focuses on the reaction between a strong acid (HCl) and a strong base (NaOH), it's important to understand that the net ionic equation can differ depending on the strength of the acid and base.
Weak Acids and Weak Bases
When a weak acid or a weak base is involved, the reaction doesn't completely dissociate into ions. Consequently, the complete ionic equation and the net ionic equation will be more complex and will show the undissociated weak acid or base molecule. The equilibrium constant, Ka or Kb, plays a vital role in determining the extent of dissociation and thus influences the net ionic equation.
Polyprotic Acids and Bases
Polyprotic acids (like sulfuric acid, H₂SO₄) and bases can donate or accept multiple protons. This leads to multiple steps in the neutralization process, resulting in more complex net ionic equations for each step. Each proton donation or acceptance will have its own net ionic equation.
Practical Considerations and Further Exploration
To fully grasp the concept, consider conducting experiments involving titrations of HCl with NaOH. Observing the pH changes as the base is added can help visualize the neutralization process.
Advanced Topics
Exploring topics like:
- Thermodynamics of Neutralization: Examining the enthalpy change (ΔH) during the reaction.
- Electrochemistry: Investigating the use of electrochemical cells to study neutralization reactions.
- Kinetics of Neutralization: Understanding the reaction rate and its dependence on various factors.
will provide a more comprehensive understanding of the reaction and its broader context within chemistry.
In conclusion, the net ionic equation for HCl and NaOH, H⁺(aq) + OH⁻(aq) → H₂O(l), represents a fundamental chemical process with significant applications across various fields. While seemingly simple, it encapsulates core principles of acid-base chemistry and provides a framework for understanding more complex neutralization reactions involving weak acids, weak bases, and polyprotic species. Understanding this reaction at the net ionic level empowers a deeper appreciation of chemistry and its real-world applications.
Latest Posts
Latest Posts
-
How Many Light Years To The Moon
Mar 25, 2025
-
What Is The Correct Order Of Phases In Cellular Respiration
Mar 25, 2025
-
Vessels That Contain Valves To Prevent Backflow Of Blood
Mar 25, 2025
-
Sr Oh 2 Strong Or Weak
Mar 25, 2025
-
What Part Of The Cell Serves As The Intracellular Highway
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation For Hcl And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
