Match Each Enzyme With The Substrate It Acts Upon
News Leon
Mar 27, 2025 · 6 min read
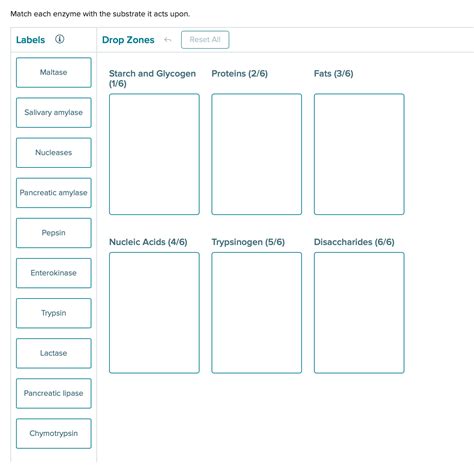
Table of Contents
Matching Enzymes with Their Substrates: A Comprehensive Guide
Enzymes are biological catalysts, crucial for virtually every biochemical reaction within living organisms. Understanding the specific substrate each enzyme acts upon is fundamental to comprehending cellular processes, metabolic pathways, and various biological functions. This article delves into the intricate relationship between enzymes and their substrates, providing a comprehensive overview of various enzyme classes and their corresponding substrates, along with illustrative examples. We'll explore the principles of enzyme-substrate specificity, factors affecting enzyme activity, and the importance of this enzyme-substrate interaction in maintaining life.
Understanding Enzyme-Substrate Specificity
The cornerstone of enzyme function lies in its specificity. This means that each enzyme is designed to interact with a specific substrate or a very limited range of substrates. This specificity is dictated by the enzyme's unique three-dimensional structure, particularly the active site. The active site is a cleft or pocket on the enzyme's surface where the substrate binds. The precise arrangement of amino acid residues within the active site ensures a complementary fit with the substrate's structure, much like a lock and key.
Several models attempt to explain this interaction:
-
Lock and Key Model: This classical model proposes a rigid active site that precisely complements the shape of the substrate. Only the correct substrate can fit into the active site, initiating the catalytic reaction.
-
Induced Fit Model: This more refined model suggests that the enzyme's active site is flexible and undergoes conformational changes upon substrate binding. The substrate binding induces a change in the active site's shape, optimizing the interaction and facilitating catalysis. This model better explains the enzyme's ability to interact with a wider range of substrates, albeit with varying efficiency.
Major Enzyme Classes and Their Substrates
Enzymes are classified into six major classes based on the type of reaction they catalyze:
1. Oxidoreductases: These enzymes catalyze oxidation-reduction reactions, involving the transfer of electrons or hydrogen atoms.
- Substrates: A wide variety of substrates can be involved, including alcohols, aldehydes, ketones, and various organic molecules. Common examples include:
- Alcohol dehydrogenase: Acts on alcohols, oxidizing them to aldehydes or ketones. The substrate is ethanol (alcohol) and the product is acetaldehyde.
- Lactate dehydrogenase: Catalyzes the interconversion of lactate and pyruvate. The substrate is lactate and pyruvate, acting as the product and substrate respectively depending on the direction of the reaction.
- Cytochrome c oxidase: A crucial enzyme in the electron transport chain, oxidizing cytochrome c and reducing oxygen to water.
2. Transferases: These enzymes catalyze the transfer of a functional group from one molecule (donor) to another (acceptor).
- Substrates: The substrates vary widely depending on the specific transferase, including amino acids, phosphate groups, methyl groups, and acyl groups. Examples include:
- Aminotransferases (transaminases): Transfer amino groups from amino acids to keto acids. For example, alanine aminotransferase (ALT) transfers the amino group from alanine to α-ketoglutarate. The substrates are Alanine and α-ketoglutarate.
- Kinases: Transfer phosphate groups from ATP to other molecules, often involved in phosphorylation cascades. Hexokinase, for instance, phosphorylates glucose to glucose-6-phosphate; the substrates are glucose and ATP.
- Acetyltransferases: Transfer acetyl groups. For example, acetyl-CoA acetyltransferase is involved in fatty acid metabolism.
3. Hydrolases: These enzymes catalyze hydrolysis reactions, breaking down molecules by adding water.
- Substrates: A vast range of molecules undergo hydrolysis, including esters, amides, peptides, and glycosidic bonds. Examples include:
- Lipases: Hydrolyze lipids (fats) into fatty acids and glycerol. Triglycerides are a common substrate.
- Proteases (peptidases): Hydrolyze peptide bonds in proteins, breaking them down into smaller peptides or amino acids. Trypsin and chymotrypsin are examples that act on specific peptide bonds in proteins.
- Amylases: Hydrolyze starch into simpler sugars like maltose. Starch is the primary substrate.
4. Lyases: These enzymes catalyze the addition or removal of groups to or from a double bond, without hydrolysis or oxidation-reduction.
- Substrates: Substrates often contain double bonds or can form double bonds during the reaction. Examples include:
- Decarboxylases: Remove carboxyl groups from molecules. Pyruvate decarboxylase removes a carboxyl group from pyruvate, forming acetaldehyde. Pyruvate is the substrate.
- Aldolases: Catalyze the cleavage of carbon-carbon bonds in aldoses and ketoses. Fructose-1,6-bisphosphate aldolase is a key enzyme in glycolysis.
5. Isomerases: These enzymes catalyze the rearrangement of atoms within a molecule, converting one isomer into another.
- Substrates: Isomers are molecules with the same chemical formula but different structural arrangements. Examples include:
- Phosphoglucose isomerase: Interconverts glucose-6-phosphate and fructose-6-phosphate. Glucose-6-phosphate is the substrate.
- Triosephosphate isomerase: Interconverts glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Glyceraldehyde-3-phosphate is the substrate.
6. Ligases (Synthetases): These enzymes catalyze the joining of two molecules, often coupled with the hydrolysis of ATP.
- Substrates: Substrates usually include two molecules that need to be joined together, and ATP, which provides the energy for the reaction. Examples include:
- DNA ligase: Joins DNA fragments, sealing breaks in the DNA backbone. DNA fragments are the substrates.
- Aminoacyl-tRNA synthetases: Attach amino acids to their corresponding tRNA molecules. Amino acids and tRNA molecules are the substrates.
Factors Affecting Enzyme Activity
Several factors influence the rate at which enzymes catalyze reactions:
-
Substrate Concentration: Increasing substrate concentration generally increases reaction rate up to a point (saturation). Beyond saturation, adding more substrate doesn't significantly increase the rate because all enzyme active sites are occupied.
-
Enzyme Concentration: Increasing enzyme concentration directly increases the reaction rate, provided there is sufficient substrate.
-
Temperature: Enzymes have an optimal temperature range; increasing temperature initially speeds up the reaction, but excessive heat can denature the enzyme, losing its activity.
-
pH: Each enzyme has an optimal pH range. Deviations from the optimal pH can alter the enzyme's structure and activity.
-
Inhibitors: Inhibitors are molecules that bind to enzymes and decrease their activity. Competitive inhibitors bind to the active site, competing with the substrate; non-competitive inhibitors bind elsewhere, altering the enzyme's shape and reducing its activity.
-
Activators: Activators are molecules that enhance enzyme activity, often by binding to the enzyme and inducing a conformational change that improves substrate binding or catalysis.
The Importance of Enzyme-Substrate Interactions
The precise interaction between enzymes and their substrates is paramount for maintaining life. These interactions are fundamental to:
-
Metabolism: Enzymes catalyze the thousands of reactions involved in metabolic pathways, enabling energy production, biosynthesis, and waste removal.
-
Signal Transduction: Enzymes play crucial roles in signal transduction cascades, amplifying and transmitting signals within cells.
-
DNA Replication and Repair: Enzymes are vital for DNA replication, repair, and transcription, ensuring the accurate transmission of genetic information.
-
Protein Synthesis: Enzymes participate in every step of protein synthesis, from transcription to translation.
-
Immune Response: Enzymes are involved in the activation and regulation of the immune system.
Conclusion
Understanding the specific substrate each enzyme acts upon is critical to comprehending the complexity of biological systems. The specificity of this enzyme-substrate interaction, governed by the enzyme's three-dimensional structure and the active site, is fundamental to the functioning of all living organisms. By studying these interactions, we gain insights into metabolic pathways, cellular processes, and disease mechanisms, paving the way for advancements in medicine, biotechnology, and other fields. Further research continues to unravel the intricate details of enzyme-substrate interactions, expanding our understanding of life's fundamental processes. This knowledge is vital for developing new drugs, therapies, and diagnostic tools, further highlighting the importance of this crucial area of biological study. The complexity of enzyme-substrate relationships extends beyond the simple lock and key model, encompassing allosteric regulation, co-factors, and intricate feedback mechanisms that maintain the delicate balance within living organisms. The exploration of these intricate details is ongoing, constantly revealing new layers of understanding and providing exciting opportunities for scientific advancement.
Latest Posts
Latest Posts
-
How To Remember Greater Than And Less Than
Mar 30, 2025
-
A Portrait Of A Lady Summary
Mar 30, 2025
-
A Consumer That Eats Both Plants And Animals
Mar 30, 2025
-
How To Separate Salt And Sand
Mar 30, 2025
-
50 Sentences Of Active And Passive Voice
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Match Each Enzyme With The Substrate It Acts Upon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
