Mass Of An Electron In Grams
News Leon
Mar 23, 2025 · 7 min read
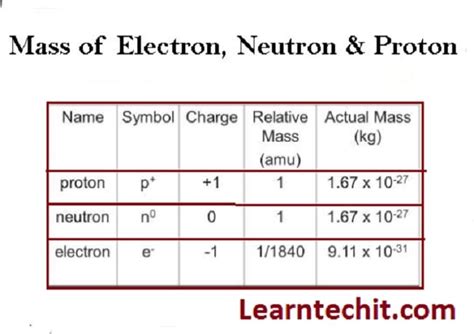
Table of Contents
The Mass of an Electron in Grams: A Deep Dive into Subatomic Physics
The electron, a fundamental constituent of matter, is a cornerstone of modern physics. While its charge is often the focus of discussions, its mass is equally crucial in understanding atomic structure, chemical reactions, and the behavior of matter at both macroscopic and microscopic scales. This article delves deep into the electron's mass, exploring its measurement, significance, and implications across various scientific fields. We'll journey from the historical context of its discovery to the precise modern measurements and the ongoing efforts to refine our understanding.
The Historical Quest to Weigh an Electron
The journey to determine the mass of an electron is a fascinating tale interwoven with the advancements in experimental physics. Unlike macroscopic objects readily weighed on a balance scale, measuring the mass of a subatomic particle requires sophisticated techniques.
Early Experiments and Estimations:
The late 19th and early 20th centuries witnessed a surge in discoveries related to cathode rays and the identification of the electron. J.J. Thomson's groundbreaking experiments, around 1897, provided the first evidence for the existence of electrons. His experiments, involving the deflection of cathode rays by electric and magnetic fields, allowed him to calculate the charge-to-mass ratio (e/m) of the electron. While this didn't directly give the mass, it laid the crucial groundwork.
It was Robert Millikan's famous oil drop experiment, in 1909, that provided the critical missing piece. By meticulously observing the motion of charged oil droplets under the influence of gravity and electric fields, Millikan determined the elementary charge (e). Combining this value with Thomson's e/m ratio allowed for the calculation of the electron's mass. The early estimations were relatively imprecise by today's standards, yet they represented a monumental leap forward.
Refining the Measurement:
The initial measurements served as a starting point for a long process of refinement. Over the decades, advancements in experimental techniques, including improved vacuum systems, more sensitive detectors, and sophisticated data analysis methods, dramatically increased the accuracy of the electron's mass determination. The development of particle accelerators and spectroscopy techniques further contributed to this refinement.
The Modern Value and its Significance
Today, the mass of an electron is incredibly well-established. It's expressed in various units, but the most relevant for this discussion is the gram. While the number itself might seem incredibly small, its implications are profound.
Mass in Grams: A Tiny but Mighty Number
The accepted value for the electron's mass is approximately 9.10938356 × 10⁻²⁸ grams. This minuscule number highlights the incredibly small scale of the subatomic world. To put it in perspective, a single grain of sand contains trillions upon trillions of electrons.
However, despite its small mass, the electron plays a vital role in determining the properties of matter. Its mass contributes to the overall mass of atoms and molecules, impacting their behavior in chemical reactions, their interactions with light, and their overall properties.
The Role of Mass in Atomic Structure and Chemical Reactions:
The electron's mass, in conjunction with its charge, dictates the electron's behavior within atoms. Electrons orbit the nucleus, held in place by the electromagnetic force. The arrangement of electrons in different energy levels dictates the chemical properties of elements and governs how atoms interact to form molecules. Understanding the electron's mass is crucial for comprehending the chemical behavior of matter.
The electron's mass influences the energy levels within an atom, as well as the energy changes associated with electronic transitions. These transitions are responsible for the emission and absorption of light, forming the basis of spectroscopy—a crucial tool for analyzing the composition and properties of matter.
Mass and Relativity:
Einstein's theory of special relativity demonstrates the equivalence of mass and energy, famously expressed by the equation E=mc². This equation shows that mass and energy are interchangeable. A small amount of mass can be converted into a tremendous amount of energy, as seen in nuclear reactions. While the electron's rest mass is small, its energy equivalent is significant in high-energy physics experiments. The relativistic effects become increasingly important when electrons are accelerated to near the speed of light.
Beyond the Mass: Other Properties of the Electron
While the mass is a fundamental property, the electron possesses other crucial characteristics that define its behavior and interaction with the universe:
Charge:
The electron carries a negative elementary charge, a fundamental constant in physics. This charge is equal in magnitude but opposite in sign to the charge of a proton. The interaction between electrons and protons is the fundamental force responsible for the structure of atoms and molecules.
Spin:
The electron possesses an intrinsic angular momentum known as spin, quantized in units of ħ/2. This spin is a quantum mechanical property and doesn't correspond to the classical idea of rotation. The electron's spin contributes to its magnetic moment, which is crucial in understanding phenomena like electron paramagnetic resonance (EPR) spectroscopy.
Wave-Particle Duality:
The electron exhibits a fascinating wave-particle duality, behaving like both a particle and a wave depending on the experimental setup. This duality is central to quantum mechanics and is crucial for understanding the behavior of electrons in atoms and molecules. The electron's wave-like properties are described by its wave function, a mathematical function that gives the probability of finding the electron at a particular location.
Measuring the Mass: Advanced Techniques
The precision with which we know the electron's mass is a testament to the advancements in experimental physics. Modern measurements involve sophisticated techniques pushing the boundaries of measurement capabilities:
Penning Traps:
Penning traps utilize a combination of electric and magnetic fields to confine charged particles. By precisely measuring the particles' cyclotron frequency and their magnetron frequency, scientists can determine their mass with exceptional accuracy. These traps are crucial for high-precision mass spectrometry, enabling the measurement of the masses of fundamental particles with unprecedented accuracy.
Quantum Electrodynamics (QED):
QED, a powerful theory that describes the interaction of light and matter, is crucial for accurately predicting the electron's properties. The theoretical predictions of QED are remarkably consistent with experimental measurements, further solidifying our understanding of the electron's fundamental properties.
Ongoing Research:
Despite the high accuracy of the current measurements, research continues to refine our knowledge of the electron's mass. Scientists are constantly exploring new techniques and pushing the boundaries of measurement precision. These efforts not only improve our understanding of the electron but also enhance our understanding of fundamental physics and the universe.
The Electron's Mass: Implications Across Scientific Fields
The electron's mass isn't just a number in a physics textbook; it has far-reaching implications across various scientific fields:
Chemistry:
As mentioned earlier, the electron's mass is critical in chemistry. It influences atomic and molecular structure, determining chemical bonds, reactivity, and the properties of matter. Understanding the electron's mass is essential for modeling chemical reactions and predicting the behavior of molecules.
Materials Science:
The behavior of electrons in materials dictates their electrical, magnetic, and optical properties. The mass of the electron plays a crucial role in determining the band structure of solids, influencing conductivity, semiconductor behavior, and other material properties.
Astrophysics and Cosmology:
The electron's mass is important in understanding the evolution of stars, galaxies, and the universe. It affects the processes of stellar nucleosynthesis, the formation of planets, and the overall dynamics of cosmic structures.
Nuclear Physics:
In nuclear physics, understanding the interaction of electrons with nuclei is crucial. Beta decay, a type of radioactive decay, involves the emission of electrons, and the mass of the electron is important for understanding this process.
Conclusion: A Tiny Particle, A Vast Impact
The seemingly insignificant mass of the electron—a minuscule fraction of a gram—holds immense significance in the grand scheme of the universe. Its mass, in conjunction with other properties, governs the behavior of matter at all scales, from the subatomic to the cosmic. The ongoing quest to refine our knowledge of the electron's mass reflects the insatiable human curiosity and the relentless pursuit of deeper understanding of the fundamental building blocks of our reality. The journey from the early estimations to the remarkably precise modern measurements showcases the power of scientific inquiry and the extraordinary precision achieved by modern experimental physics. The electron, despite its tiny mass, continues to play a starring role in shaping our understanding of the cosmos.
Latest Posts
Latest Posts
-
Select The Correct Iupac Name For The Following Compound
Mar 25, 2025
-
How Many Atoms Are In A Single Molecule Of Water
Mar 25, 2025
-
Electric Field Infinite Line Of Charge
Mar 25, 2025
-
A Pure Substance Containing Only One Kind Of
Mar 25, 2025
-
A Species Is A Group Of Organisms That
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Mass Of An Electron In Grams . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
