Lactose Is The Substrate Of Which Enzyme
News Leon
Mar 24, 2025 · 6 min read
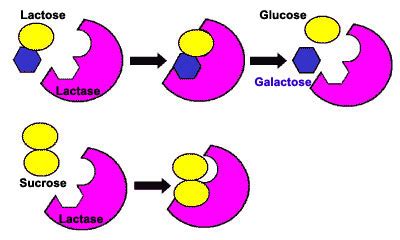
Table of Contents
Lactose: The Substrate of β-Galactosidase
Lactose, the primary sugar found in milk, isn't just a simple carbohydrate; it's a key player in various biological processes, and its metabolism hinges on a specific enzyme: β-galactosidase. This article delves deep into the relationship between lactose and β-galactosidase, exploring their chemical structures, the enzymatic reaction they participate in, the regulation of β-galactosidase production, and the broader implications for human health and industrial applications.
Understanding Lactose: Structure and Properties
Lactose, also known as milk sugar, is a disaccharide – a sugar composed of two monosaccharides linked together. Specifically, it's a β-galactoside, meaning it consists of a galactose molecule and a glucose molecule joined by a β-1,4-glycosidic bond. This bond is crucial because it dictates how β-galactosidase interacts with lactose. The structure of lactose isn't just a simple chain; it possesses specific conformational properties influencing its solubility, digestibility, and interaction with enzymes.
Chemical Structure: The precise arrangement of atoms within the galactose and glucose units, along with the orientation of the β-1,4-glycosidic bond, defines lactose's unique chemical identity. This structure is recognized and bound by β-galactosidase, initiating the enzymatic breakdown.
Properties: Lactose exhibits various properties, including its solubility in water (although less soluble than sucrose), its ability to crystallize, and its role in determining the texture and sweetness of dairy products. These properties are all indirectly related to its metabolism via β-galactosidase. For example, the incomplete breakdown of lactose can lead to the formation of crystalline structures in certain dairy products.
β-Galactosidase: The Enzyme That Breaks Down Lactose
β-Galactosidase (also known as lactase) is a hydrolase enzyme; it catalyzes the hydrolysis of lactose into its constituent monosaccharides: glucose and galactose. This hydrolytic reaction is essential for the digestion and utilization of lactose by organisms. Without functional β-galactosidase, lactose passes undigested through the digestive system, leading to various symptoms associated with lactose intolerance.
Mechanism of Action: β-galactosidase operates through a precise mechanism. The enzyme possesses an active site specifically designed to bind to the lactose molecule. This binding induces a conformational change in the enzyme, creating an optimal environment for the hydrolysis reaction. A water molecule participates in the reaction, breaking the β-1,4-glycosidic bond and liberating glucose and galactose. The details of this mechanism, involving acid-base catalysis and transition state stabilization, are complex and continue to be areas of active research.
Specificity: β-Galactosidase shows high substrate specificity, primarily acting on β-galactosides like lactose. However, it may exhibit some activity against other substrates with similar structural features, though generally at a much lower rate. This specificity is crucial to its function in maintaining the metabolic balance within cells.
Location and Sources: β-Galactosidase is found in various organisms, including bacteria, fungi, and mammals. In mammals, it's predominantly located in the small intestine, where lactose digestion occurs. Different organisms produce variations of β-galactosidase, each with subtle differences in its properties and optimal operating conditions. Bacterial sources are commonly used for industrial production of the enzyme.
Regulation of β-Galactosidase Production: An Operon Model
In bacteria like E. coli, the production of β-galactosidase is tightly regulated by an operon system – the lac operon. This system ensures that the enzyme is produced only when lactose is present and glucose is scarce.
The Lac Operon: The lac operon comprises three structural genes (lacZ, lacY, and lacA), encoding β-galactosidase, lactose permease (which transports lactose into the cell), and thiogalactoside transacetylase (whose function is less clear), respectively. The operon's expression is controlled by regulatory elements such as the promoter, operator, and repressor protein.
Regulation by Repressor Protein: In the absence of lactose, a repressor protein binds to the operator region, preventing RNA polymerase from transcribing the structural genes. When lactose is present, it acts as an inducer, binding to the repressor protein and causing a conformational change that prevents it from binding to the operator, thereby allowing transcription of the lac operon genes.
Catabolite Repression: Even in the presence of lactose, the lac operon's expression is further regulated by the presence of glucose. When glucose is abundant, catabolite repression occurs, reducing the expression of the lac operon. This ensures that the cell preferentially utilizes glucose, a more efficient energy source, before resorting to lactose metabolism.
Lactose Intolerance and β-Galactosidase Deficiency
Lactose intolerance, a common condition, results from a deficiency or reduced activity of β-galactosidase in the small intestine. This deficiency prevents the efficient breakdown of lactose, leading to various symptoms like bloating, gas, abdominal cramps, and diarrhea. The severity of symptoms varies depending on the level of enzyme deficiency and the amount of lactose consumed.
Causes of Lactose Intolerance: Lactose intolerance can stem from various factors, including genetic predisposition, age-related decline in enzyme production (lactase persistence is less common in many adult populations), and intestinal diseases. The underlying cause dictates the appropriate management strategy.
Diagnosis and Management: Lactose intolerance is typically diagnosed through a combination of symptom assessment, lactose tolerance tests, and sometimes genetic testing. Management strategies focus on dietary modifications, limiting lactose consumption or supplementing with β-galactosidase enzymes in the form of lactase tablets or drops.
Industrial Applications of β-Galactosidase
β-Galactosidase finds extensive applications in various industries, mainly due to its ability to hydrolyze lactose and its diverse catalytic capabilities.
Dairy Industry: The enzyme is used to reduce lactose content in dairy products, making them more digestible for lactose-intolerant individuals. It's also utilized in the production of whey, a byproduct of cheese-making, which can be further processed into valuable ingredients. Moreover, the hydrolysis of lactose yields glucose and galactose, which can be used as sweeteners or substrates for other industrial processes.
Pharmaceutical Industry: β-Galactosidase is involved in producing various pharmaceuticals, often by modifying lactose or other galactosides. Its ability to attach other molecules to lactose makes it useful in bioconjugation and drug delivery applications.
Other Applications: β-galactosidase also plays a role in various other industrial processes:
- Biofuel Production: Its action on lactose in whey contributes to the generation of bioethanol.
- Food and Beverage Industry: Improving the texture and digestibility of foods.
- Analytical Chemistry: Acting as a probe for detecting and measuring galactosides.
Future Research Directions
Despite the extensive knowledge about lactose and β-galactosidase, ongoing research continues to explore various aspects:
- Developing more efficient and stable β-galactosidase variants: This involves protein engineering to improve enzyme properties such as activity, stability, and tolerance to various conditions.
- Understanding the precise mechanisms of enzyme regulation: Further research is necessary to fully elucidate the intricate control mechanisms governing β-galactosidase production and activity.
- Exploring novel applications: The potential of β-galactosidase in new areas, such as targeted drug delivery and biosynthesis of valuable compounds, is actively being investigated.
- Addressing lactose intolerance: Research focuses on developing effective therapies and preventative strategies for lactose intolerance.
Conclusion
The relationship between lactose and β-galactosidase is fundamental to the understanding of lactose metabolism, both in biological systems and industrial processes. The enzyme's specificity for lactose, its regulated production, and its broad applications make it a subject of continuous interest in various fields. Further research promises to unlock even more possibilities for leveraging the power of β-galactosidase for human benefit. From addressing lactose intolerance to driving innovation in biofuel and pharmaceutical industries, the future of β-galactosidase research is indeed promising.
Latest Posts
Latest Posts
-
How Many Atoms Are In Sodium
Mar 26, 2025
-
The Urinary Bladder Is Composed Of What Epithelium
Mar 26, 2025
-
Sphere Is To Circle As Cube Is To
Mar 26, 2025
-
A Convex Lens Of Focal Length 10cm
Mar 26, 2025
-
Gases Have Indefinite Shape And Volume
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Lactose Is The Substrate Of Which Enzyme . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
