How Many Atoms Are In Sodium
News Leon
Mar 26, 2025 · 5 min read
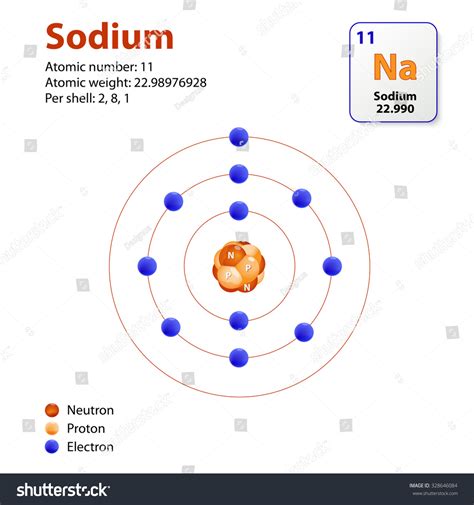
Table of Contents
How Many Atoms Are in Sodium? A Deep Dive into Avogadro's Number and Atomic Mass
Determining the exact number of atoms in a given amount of sodium isn't as straightforward as it might seem. It requires understanding fundamental concepts in chemistry, particularly Avogadro's number and molar mass. This article will explore these concepts in detail, providing a comprehensive answer to the question: how many atoms are there in sodium, and how do we calculate that number for various quantities of the element?
Understanding the Fundamentals: Moles, Avogadro's Number, and Atomic Mass
Before we can tackle the question of atom count, we need to grasp three key concepts:
1. The Mole (mol): The Chemist's Counting Unit
Imagine trying to count grains of sand on a beach – it's impossible! Chemists face a similar challenge when dealing with atoms and molecules, as their numbers are astronomically large. This is where the mole comes in. A mole is simply a unit representing a specific number of particles – Avogadro's number (approximately 6.022 x 10²³). One mole of any substance contains Avogadro's number of elementary entities (atoms, molecules, ions, etc.). It's a convenient way to handle massive quantities of tiny particles.
2. Avogadro's Number: The Bridge Between the Macroscopic and Microscopic
Avogadro's number is a fundamental constant in chemistry. It's the number of atoms in exactly 12 grams of carbon-12. This seemingly arbitrary number acts as a crucial conversion factor, allowing us to relate the macroscopic world (grams, kilograms) to the microscopic world (atoms, molecules). It's the link between the weight we can measure and the number of atoms present.
3. Atomic Mass: Weighting the Atom
The atomic mass of an element is the average mass of all its isotopes, weighted by their relative abundance. Sodium (Na) has an atomic mass of approximately 22.99 atomic mass units (amu). This means a single sodium atom weighs, on average, 22.99 amu. Crucially, one mole of sodium atoms weighs approximately 22.99 grams. This relationship is the cornerstone of our calculations.
Calculating the Number of Atoms in Sodium: A Step-by-Step Approach
Now, let's address the central question: how many atoms are in a specific amount of sodium? The process involves using the concepts we've just discussed:
1. Specify the Amount of Sodium: Let's assume we have 1 gram of sodium.
2. Convert Grams to Moles: We use the molar mass of sodium (approximately 22.99 g/mol).
* Moles of sodium = (mass of sodium in grams) / (molar mass of sodium)
* Moles of sodium = 1 g / 22.99 g/mol ≈ 0.0435 moles
3. Convert Moles to Atoms: We use Avogadro's number (6.022 x 10²³) to convert moles to the number of atoms.
* Number of sodium atoms = (moles of sodium) x (Avogadro's number)
* Number of sodium atoms ≈ 0.0435 moles x 6.022 x 10²³ atoms/mol ≈ 2.62 x 10²² atoms
Therefore, in 1 gram of sodium, there are approximately 2.62 x 10²² atoms.
Exploring Different Quantities of Sodium
The above calculation can be adapted to find the number of atoms in any amount of sodium. Let's consider a few examples:
Example 1: 10 grams of Sodium
- Moles of sodium = 10 g / 22.99 g/mol ≈ 0.435 moles
- Number of sodium atoms ≈ 0.435 moles x 6.022 x 10²³ atoms/mol ≈ 2.62 x 10²³ atoms
Example 2: 1 mole of Sodium
- This is straightforward: 1 mole of sodium contains Avogadro's number of atoms, approximately 6.022 x 10²³ atoms.
Example 3: A macroscopic quantity – 1 kilogram of Sodium
- Moles of sodium = 1000 g / 22.99 g/mol ≈ 43.5 moles
- Number of sodium atoms ≈ 43.5 moles x 6.022 x 10²³ atoms/mol ≈ 2.62 x 10²⁵ atoms
Beyond the Basics: Isotopes and Atomic Mass Precision
The calculations above use the average atomic mass of sodium. However, sodium has isotopes (atoms with the same number of protons but different numbers of neutrons). These isotopes have slightly different masses. If we need a more precise calculation, we would need to consider the isotopic composition of the sodium sample. This would involve knowing the relative abundance of each sodium isotope and using their individual masses to calculate a weighted average atomic mass for that specific sample. This level of precision is often unnecessary in most situations.
Practical Applications and Significance
Understanding how to calculate the number of atoms in a given amount of a substance has far-reaching implications across various fields:
-
Chemistry: Stoichiometry, chemical reactions, and quantitative analysis heavily rely on these calculations. Understanding the relationship between mass, moles, and atoms is crucial for predicting the outcome of chemical processes.
-
Materials Science: The properties of materials depend on the arrangement and interaction of their constituent atoms. Knowing the number of atoms in a sample allows for a deeper understanding of material behaviour and design.
-
Nuclear Physics: Radioactive decay processes, nuclear reactions, and nuclear energy calculations require precise knowledge of the number of atoms involved.
Conclusion: A Journey into the Atomic Realm
The question of how many atoms are in sodium might seem simple at first glance. However, the answer unveils a rich understanding of fundamental chemical concepts like moles, Avogadro's number, and atomic mass. Mastering these concepts is vital for anyone pursuing a deeper understanding of chemistry and its applications. While the number of atoms in a specific quantity of sodium can be calculated relatively easily, the underlying principles and their broad implications extend far beyond this simple calculation, highlighting the intricate and fascinating world of atoms and molecules.
Latest Posts
Latest Posts
-
What Continent Is Closest To Antarctica
Mar 29, 2025
-
What Is 0 07 As A Percentage
Mar 29, 2025
-
How Many Unpaired Electrons Does Oxygen Have
Mar 29, 2025
-
What Is Not True About The Cell Membrane
Mar 29, 2025
-
Example Of Request Letter For Permission
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
