Label The Parts Of The Immunoglobulin
News Leon
Mar 25, 2025 · 6 min read
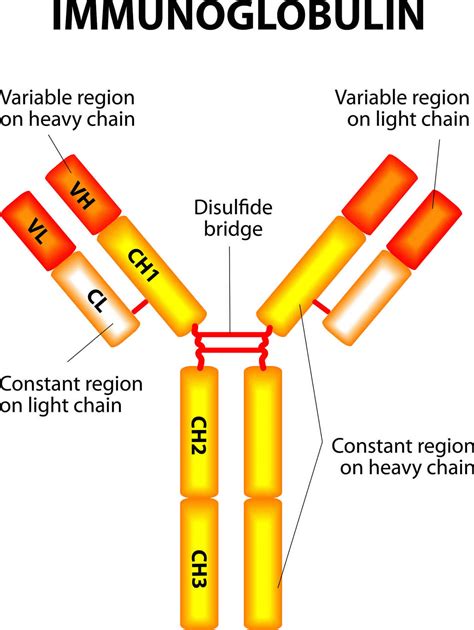
Table of Contents
Labeling the Parts of an Immunoglobulin: A Deep Dive into Antibody Structure
Immunoglobulins (Ig), also known as antibodies, are glycoprotein molecules produced by plasma cells (white blood cells). They play a crucial role in the adaptive immune system, recognizing and neutralizing foreign substances like bacteria, viruses, fungi, and toxins. Understanding the structure of immunoglobulins is essential for comprehending their function and the mechanisms of humoral immunity. This article provides a detailed exploration of immunoglobulin structure, labeling its key components and explaining their roles.
The Basic Structure of Immunoglobulins: A Y-Shaped Molecule
Immunoglobulins share a basic Y-shaped structure, composed of four polypeptide chains: two identical heavy chains (H chains) and two identical light chains (L chains). These chains are linked together by disulfide bonds, creating a highly stable yet flexible molecule. The four chains are further subdivided into distinct regions:
1. Variable Regions (V regions): The Key to Antigen Specificity
The variable regions of both the heavy and light chains (VH and VL) are located at the tips of the "Y" structure. This is the antigen-binding site, also known as the paratope. The unique amino acid sequence within these regions determines the specific antigen that the antibody can bind to. This high level of variability allows the immune system to produce a vast repertoire of antibodies capable of recognizing a multitude of different antigens.
Key features of the variable regions:
- Hypervariable regions (complementarity-determining regions or CDRs): Within the V regions are three highly variable segments called hypervariable regions or CDRs (CDR1, CDR2, and CDR3). These CDRs form the actual contact points with the antigen, exhibiting remarkable diversity in amino acid sequence.
- Framework regions (FRs): The more conserved regions between the CDRs are called framework regions (FR1, FR2, FR3, and FR4). These regions provide a structural scaffold for the CDRs, ensuring their proper positioning and orientation for antigen binding. The framework regions maintain the overall structure of the V region.
2. Constant Regions (C regions): Effectors of the Immune Response
The constant regions of the heavy and light chains (CH and CL) are relatively conserved in amino acid sequence within a given antibody isotype. These regions don't participate directly in antigen binding, but they are crucial for mediating the effector functions of the antibody. The constant region of the heavy chain determines the antibody isotype.
Antibody Isotypes: Defining the Functional Class
The five major isotypes of immunoglobulins in humans are:
- IgG: The most abundant isotype in serum, mediating various effector functions including opsonization (enhancing phagocytosis), complement activation, and antibody-dependent cell-mediated cytotoxicity (ADCC). There are four subclasses of IgG (IgG1, IgG2, IgG3, and IgG4) with slightly different properties.
- IgM: The first antibody produced during an immune response, typically found as a pentamer (five IgM monomers joined together). Highly efficient at activating complement.
- IgA: The predominant antibody isotype in mucosal secretions (e.g., saliva, tears, breast milk), providing protection against pathogens at mucosal surfaces. Exists as a monomer or dimer.
- IgD: Its function is not fully understood, but it may play a role in B cell activation and differentiation.
- IgE: Involved in allergic reactions and defense against parasitic infections. Binds to mast cells and basophils, triggering the release of histamine and other inflammatory mediators upon antigen binding.
Detailed Breakdown of Immunoglobulin Regions: A Closer Look
Heavy Chain Structure: Defining the Isotype and Function
The heavy chain is the defining feature distinguishing the different antibody isotypes. The constant region of the heavy chain (CH) is further divided into several domains:
- CH1: The first constant region domain, adjacent to the variable region.
- CH2: This domain plays a crucial role in complement activation and Fc receptor binding (for interaction with immune cells).
- CH3: Also involved in Fc receptor binding and other effector functions.
- CH4 (in some isotypes): Present only in IgG and IgE, contributing to effector functions.
The hinge region, located between CH1 and CH2, provides flexibility to the antibody molecule, allowing it to bind to antigens with different spacing and orientations.
Light Chain Structure: Two Main Types
There are two main types of light chains: kappa (κ) and lambda (λ). Both types have a variable region (VL) and a constant region (CL), with the constant region being less diverse than the heavy chain constant region. Each antibody molecule carries either two kappa or two lambda light chains, but not a mixture of both.
The Fc Region: The Effector Arm of the Antibody
The Fc region (fragment crystallizable) encompasses the carboxy-terminal portions of the two heavy chains, excluding the variable regions. This region is crucial for the effector functions of the antibody, interacting with various immune cells and molecules to trigger a variety of downstream effects. Key aspects of the Fc region include:
- Fc receptor (FcR) binding: The Fc region binds to specific receptors on immune cells like macrophages, neutrophils, and NK cells. This binding triggers processes like phagocytosis, ADCC, and antibody-dependent cellular phagocytosis (ADCP). The specific FcR that an antibody binds to depends on its isotype.
- Complement activation: Certain antibody isotypes (especially IgM and IgG) can trigger the complement cascade, a series of enzymatic reactions leading to the lysis of pathogens and the enhancement of inflammation. The CH2 domain of the heavy chain is involved in this process.
- Half-life in circulation: The Fc region influences the antibody's half-life in the bloodstream.
Beyond the Basic Structure: Glycosylation and Other Modifications
The immunoglobulin structure is not static; it undergoes several post-translational modifications, including glycosylation. Glycosylation is the process of adding carbohydrate chains to specific asparagine residues in the Fc region. These glycosylations significantly impact antibody effector function. Variations in glycosylation patterns can influence antibody half-life, FcR binding affinity, and complement activation.
Technological Advances in Immunoglobulin Research
Recent advances in technology, such as single-cell sequencing, have provided researchers with powerful tools to analyze the diversity and complexity of the antibody repertoire. Understanding the intricate details of immunoglobulin structure has enabled the development of antibody engineering technologies, enabling the production of therapeutic antibodies with enhanced properties.
Applications of Understanding Immunoglobulin Structure
The detailed knowledge of immunoglobulin structure has numerous applications in medicine and biotechnology:
- Development of therapeutic antibodies: Understanding the structure allows for the design of antibodies with improved efficacy, specificity, and reduced side effects.
- Diagnostics: Antibodies are widely used as diagnostic tools to detect antigens and biomarkers associated with diseases.
- Vaccine development: Immunoglobulin structure knowledge helps in designing effective vaccines.
- Immunotherapy: Immunoglobulin-based therapies are used to treat various diseases, including cancers and autoimmune disorders.
- Research tools: Antibodies serve as crucial research tools for investigating various biological processes.
Conclusion: A Dynamic and Versatile Molecule
Immunoglobulins are remarkable molecules exhibiting exceptional diversity and specificity in antigen recognition and remarkable versatility in their effector functions. The detailed understanding of their structure, encompassing the variable and constant regions, the different isotypes, and the influence of post-translational modifications, is crucial for understanding the intricacies of the immune system and for developing innovative applications in medicine and biotechnology. Further research will undoubtedly reveal even more details about this dynamic and versatile molecule, providing further insights into its diverse roles in health and disease.
Latest Posts
Latest Posts
-
Which Biogeochemical Cycle Does Not Have An Atmospheric Component
Mar 26, 2025
-
Which Particle Is Found In The Nucleus Of The Atom
Mar 26, 2025
-
Two Long Straight Wires Are Separated By 0 120 M
Mar 26, 2025
-
Who Was The First Person To See Cells
Mar 26, 2025
-
4 Functions Of A Political Party
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Label The Parts Of The Immunoglobulin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
