Which Particle Is Found In The Nucleus Of The Atom
News Leon
Mar 26, 2025 · 6 min read
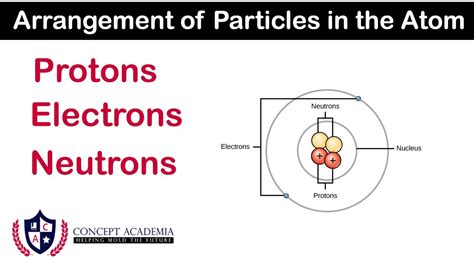
Table of Contents
Which Particle is Found in the Nucleus of the Atom? A Deep Dive into Atomic Structure
The atom, the fundamental building block of matter, has captivated scientists for centuries. Understanding its structure is crucial to comprehending the behavior of matter and the universe itself. A key aspect of this understanding lies in identifying the particles that reside within the atom's nucleus. This article will delve into the fascinating world of atomic structure, focusing specifically on the particles found within the nucleus – protons and neutrons. We'll explore their properties, their roles in determining an atom's characteristics, and the implications of their interactions.
The Nucleus: The Atom's Core
Before diving into the specific particles, it's important to establish the context of the atom's nucleus. The atom is often visualized as a miniature solar system, with a central, dense core – the nucleus – surrounded by orbiting electrons. However, this analogy, while helpful for visualizing the structure, is somewhat simplistic. The reality is far more complex and governed by quantum mechanics.
The nucleus, despite occupying a tiny fraction of the atom's overall volume, contains nearly all of its mass. This concentration of mass is due to the presence of protons and neutrons, which are significantly more massive than electrons. The strong nuclear force, a fundamental force of nature, holds these particles together within the nucleus, overcoming the electrostatic repulsion between the positively charged protons.
Protons: The Positively Charged Core
Protons are one of the two types of nucleons, the particles that constitute the atomic nucleus. They carry a positive electrical charge, equal in magnitude but opposite in sign to the charge of an electron. This positive charge is crucial for several reasons:
-
Defining Atomic Number: The number of protons in an atom's nucleus defines its atomic number. This number uniquely identifies an element on the periodic table. For example, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on. The atomic number is a fundamental property of an element, dictating its chemical behavior and properties.
-
Electrostatic Interactions: The positive charge of protons plays a crucial role in the atom's interaction with other atoms and molecules. It governs the formation of chemical bonds, which are responsible for the vast array of compounds found in the universe. The electrostatic attraction between the positively charged protons and the negatively charged electrons holds the atom together.
-
Nuclear Stability: The number of protons in the nucleus influences its stability. Nuclei with an unstable proton-neutron ratio are prone to radioactive decay, emitting particles or energy to achieve a more stable configuration.
-
Mass Contribution: Protons contribute significantly to the atom's overall mass. Their mass is approximately 1 atomic mass unit (amu), which is very close to the mass of a neutron.
Neutrons: The Neutral Partners
Neutrons, the other type of nucleon, are electrically neutral, possessing no net electrical charge. Their presence in the nucleus is crucial for several reasons:
-
Nuclear Stability: Neutrons play a vital role in nuclear stability. They help to mitigate the electrostatic repulsion between protons, enhancing the strong nuclear force that holds the nucleus together. The optimal ratio of protons to neutrons varies depending on the element, with heavier elements generally requiring a higher proportion of neutrons for stability. Isotopes, which are atoms of the same element with differing numbers of neutrons, showcase this effect. Some isotopes are stable, while others are radioactive due to an imbalanced proton-neutron ratio.
-
Mass Contribution: Similar to protons, neutrons contribute significantly to the atom's mass. Their mass is also approximately 1 amu.
-
Nuclear Reactions: Neutrons are highly effective in initiating nuclear reactions, particularly nuclear fission. Their lack of charge allows them to penetrate the nucleus easily without experiencing significant electrostatic repulsion, triggering chain reactions that release vast amounts of energy.
-
Isotope Variations: The number of neutrons in an atom's nucleus determines its isotopic form. Different isotopes of the same element have the same number of protons but varying numbers of neutrons, resulting in different mass numbers. This variation in neutron number affects the stability and properties of the isotopes.
The Strong Nuclear Force: The Glue that Holds it Together
The incredible density of the nucleus and the presence of positively charged protons raise a fundamental question: what keeps the protons from repelling each other and causing the nucleus to fly apart? The answer lies in the strong nuclear force, one of the four fundamental forces of nature.
The strong nuclear force is a short-range force that is significantly stronger than the electromagnetic force at the distances involved within the nucleus. It acts between nucleons (protons and neutrons), binding them together despite the electrostatic repulsion between protons. This force is responsible for the stability of most atomic nuclei. However, its short range explains why nuclei beyond a certain size become increasingly unstable and prone to radioactive decay.
Beyond Protons and Neutrons: Subatomic Particles
While protons and neutrons are the primary constituents of the nucleus, they are themselves composed of even smaller particles called quarks. Protons and neutrons each contain three quarks:
- Protons: consist of two up quarks and one down quark (uud).
- Neutrons: consist of one up quark and two down quarks (udd).
Quarks are fundamental particles, meaning they are not composed of any smaller known constituents. They interact through the strong force, mediated by gluons. The properties of quarks, their interactions, and the resulting properties of protons and neutrons are complex subjects studied in particle physics. Understanding quarks requires delving into the Standard Model of particle physics, a sophisticated theoretical framework explaining the fundamental building blocks of matter and their interactions.
The Significance of Nuclear Structure
The structure of the nucleus, and the properties of protons and neutrons, has profound implications across various scientific disciplines:
-
Nuclear Chemistry: The study of nuclear reactions and radioactive decay, crucial for applications like nuclear energy, radioisotope dating, and medical imaging.
-
Nuclear Physics: The investigation of the nucleus's properties and interactions, contributing to our understanding of fundamental forces and the structure of matter.
-
Astrophysics: Understanding nuclear processes is vital for modeling stellar evolution, nucleosynthesis (the formation of elements in stars), and understanding the behavior of matter in extreme environments like neutron stars.
-
Materials Science: Nuclear structure influences the properties of materials, impacting their strength, reactivity, and other characteristics. This is especially relevant in developing new materials with specific functionalities.
Conclusion: A Journey into the Heart of Matter
The nucleus, with its complement of protons and neutrons, stands as a testament to the complexity and elegance of the natural world. The properties of these particles, their interactions, and the forces that govern their behavior are fundamental to understanding the structure and behavior of matter at its most basic level. From the formation of chemical bonds to the power of nuclear reactions, the particles found in the atom's nucleus play a crucial role in shaping our world and the universe around us. Further research into the nuances of nuclear structure continues to unveil new insights, pushing the boundaries of our understanding and opening avenues for technological advancements across numerous fields. The exploration of the nucleus remains a vibrant and dynamic area of scientific inquiry, with ongoing discoveries continuously refining our understanding of the fundamental building blocks of matter.
Latest Posts
Latest Posts
-
When A Cell Is Placed In A Hypertonic Solution
Mar 28, 2025
-
How Many Neutrons Are In Sulfur
Mar 28, 2025
-
Is Celsius The Same As Centigrade
Mar 28, 2025
-
X 3 3x 2 3x 1
Mar 28, 2025
-
Which Two Microscopes Generate Three Dimensional Images
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Particle Is Found In The Nucleus Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
