Kelvin Planck Second Law Of Thermodynamics
News Leon
Mar 24, 2025 · 6 min read
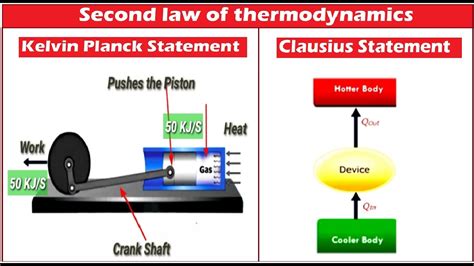
Table of Contents
Kelvin-Planck Statement of the Second Law of Thermodynamics: A Deep Dive
The Second Law of Thermodynamics is a cornerstone of physics, dictating the direction of natural processes and imposing limitations on energy conversion. While several statements encapsulate this law, the Kelvin-Planck statement offers a particularly insightful perspective on the impossibility of creating a perpetual motion machine of the second kind. This article will explore the Kelvin-Planck statement in detail, examining its implications, applications, and relationship to other formulations of the second law.
Understanding the Kelvin-Planck Statement
The Kelvin-Planck statement, also known as the Lord Kelvin statement, asserts the following:
It is impossible to devise a cyclically operating device, the sole effect of which is to absorb energy in the form of heat from a single thermal reservoir and deliver an equivalent amount of work.
Let's break this down:
-
Cyclically operating device: This refers to a system that undergoes a series of processes, returning to its initial state at the end of each cycle. This eliminates any changes in internal energy that could contribute to work output.
-
Sole effect: This crucial phrase highlights that the device cannot have any other effect besides absorbing heat and producing work. There must be no other energy transfers or changes in the system.
-
Single thermal reservoir: The heat must be absorbed from only one source. This contrasts with heat engines that operate between two reservoirs – a high-temperature source and a low-temperature sink.
-
Equivalent amount of work: The amount of work produced must equal the amount of heat absorbed. This condition rules out any possibility of spontaneous work creation from a single heat source.
In essence, the Kelvin-Planck statement declares that it's impossible to build a heat engine that extracts heat from a single reservoir and converts it entirely into work without any other effects. This directly refutes the concept of a perpetual motion machine of the second kind, a hypothetical device that violates the second law.
Perpetual Motion Machines of the Second Kind
A perpetual motion machine of the second kind (PMM2) is a hypothetical device that continuously produces work by extracting heat from a single thermal reservoir. Such a machine would seemingly violate the Kelvin-Planck statement and the second law in general. Many attempts have been made to create PMM2s, all ultimately failing due to the fundamental limitations imposed by thermodynamics. The inherent inefficiency of converting heat completely into work is a key principle underlying the impossibility of PMM2s.
Implications of the Kelvin-Planck Statement
The Kelvin-Planck statement has profound implications across various fields:
-
Limitations on Heat Engine Efficiency: The statement directly limits the efficiency of any heat engine. No heat engine can convert 100% of the heat it absorbs into work. A portion of the heat must always be rejected to a lower-temperature reservoir. This leads to the concept of Carnot efficiency, which sets an upper bound on the efficiency of any heat engine operating between two given temperatures.
-
Refrigeration and Heat Pumps: The statement also impacts the operation of refrigerators and heat pumps. These devices transfer heat from a cold reservoir to a hot reservoir, requiring external work input. The statement explains why this work input is essential – it's impossible to transfer heat spontaneously from cold to hot without any external effort.
-
Entropy and Irreversibility: The Kelvin-Planck statement is intimately linked to the concept of entropy. Entropy is a measure of disorder or randomness in a system. The second law states that the total entropy of an isolated system can only increase over time or remain constant in ideal cases (reversible processes). The impossibility of a PMM2 highlights the irreversibility of natural processes and the inevitable increase in entropy.
-
Power Generation and Energy Conservation: The limitations imposed by the Kelvin-Planck statement are crucial in the design and optimization of power generation systems. Engineers must consider the efficiency limits imposed by the second law when designing power plants and other energy conversion technologies. Understanding these limitations is essential for developing sustainable and efficient energy solutions.
Relationship to Other Statements of the Second Law
The Kelvin-Planck statement is equivalent to other formulations of the second law of thermodynamics, including the Clausius statement. The Clausius statement focuses on the impossibility of a spontaneous transfer of heat from a colder body to a hotter body without external work. Although seemingly different, both statements ultimately express the same fundamental principle: natural processes tend towards increasing entropy. The equivalence of these statements demonstrates the robustness and fundamental nature of the second law.
Applications of the Kelvin-Planck Statement
The implications of the Kelvin-Planck statement extend far beyond theoretical considerations, finding practical applications in various fields:
-
Power Plant Design: Power plant engineers use the Kelvin-Planck statement to optimize the efficiency of steam turbines, internal combustion engines, and other energy conversion devices. By understanding the limitations on heat-to-work conversion, engineers can design more efficient systems that minimize energy waste.
-
Refrigeration and Air Conditioning: The principles underlying the Kelvin-Planck statement are crucial in designing efficient refrigeration and air conditioning systems. These systems work by transferring heat against its natural flow, and understanding the thermodynamic limitations helps in designing systems that minimize energy consumption.
-
Chemical Processes: The second law of thermodynamics, encapsulated in statements like Kelvin-Planck, plays a vital role in optimizing chemical processes. Understanding the limitations on energy conversion can help in designing efficient and sustainable chemical reactions.
-
Material Science: The development of new materials with improved thermodynamic properties is influenced by the second law. Researchers are constantly striving to create materials that facilitate more efficient energy conversion and storage, pushing the boundaries of what's thermodynamically possible while staying within the constraints of the second law.
Conclusion: The Enduring Significance of the Kelvin-Planck Statement
The Kelvin-Planck statement is a powerful and concise expression of the second law of thermodynamics. It clarifies the fundamental impossibility of creating a perpetual motion machine of the second kind and underscores the irreversibility of natural processes. Its implications extend across numerous scientific and engineering disciplines, shaping our understanding of energy conversion, efficiency limits, and the nature of entropy. As we continue to grapple with challenges related to energy production and conservation, a thorough understanding of the Kelvin-Planck statement remains essential for developing innovative and sustainable solutions for the future. The statement's enduring significance lies in its ability to provide a clear and fundamental framework for analyzing and understanding the limitations and possibilities within the world of thermodynamics. Further research into the intricacies of the second law and its various statements continues to unveil deeper insights into the workings of the universe and informs advancements in technology across various fields.
Latest Posts
Latest Posts
-
Inventory Is Classified On The Balance Sheet As A
Mar 26, 2025
-
What Is The Opposite Of Hyperbole
Mar 26, 2025
-
Major And Minor Grooves In Dna
Mar 26, 2025
-
Tendons And Ligaments Are Examples Of
Mar 26, 2025
-
A Homogeneous Mixture Of Two Or More Substances
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Kelvin Planck Second Law Of Thermodynamics . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
