Is The Melting Point Of Covalent Compounds High Or Low
News Leon
Mar 17, 2025 · 5 min read
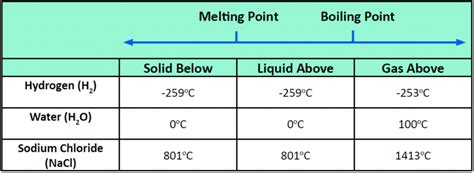
Table of Contents
Is the Melting Point of Covalent Compounds High or Low? A Comprehensive Guide
The melting point of a compound, the temperature at which it transitions from a solid to a liquid state, is a crucial physical property. It provides valuable insights into the strength of the intermolecular forces holding the molecules together. While ionic compounds are renowned for their high melting points, the melting points of covalent compounds exhibit a wider range, leading to the question: are they generally high or low? The answer, as we'll explore, is it depends.
Understanding Covalent Bonds and Intermolecular Forces
Before diving into melting points, let's establish a fundamental understanding. Covalent compounds are formed when atoms share electrons to achieve a stable electron configuration. This sharing creates strong intramolecular forces, the bonds within the molecule. However, the melting point isn't determined by these strong intramolecular bonds alone. Instead, it's primarily dictated by the intermolecular forces – the forces of attraction between molecules. These are significantly weaker than covalent bonds.
Several types of intermolecular forces exist, with varying strengths:
-
London Dispersion Forces (LDFs): Present in all molecules, these are weak forces arising from temporary, instantaneous dipoles. The larger and more complex the molecule, the stronger the LDFs.
-
Dipole-Dipole Forces: Occur in polar molecules, where there's an uneven distribution of electron density, creating permanent dipoles. These are stronger than LDFs.
-
Hydrogen Bonding: A special type of dipole-dipole interaction involving hydrogen bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). Hydrogen bonds are the strongest type of intermolecular force.
Factors Influencing the Melting Point of Covalent Compounds
The melting point of a covalent compound is a delicate balance between the strength of these intermolecular forces and the size and shape of the molecule. Several key factors come into play:
1. Molecular Size and Surface Area:
Larger molecules generally have higher melting points. This is because they possess a larger surface area, leading to more points of contact and stronger London Dispersion Forces. More interactions mean more energy is required to overcome them and transition to the liquid state. Think of it like Velcro – more hooks and loops mean a stronger attachment.
Example: Consider a series of alkanes (hydrocarbons with single bonds). As the number of carbon atoms increases (and thus the molecular size), the melting point also increases.
2. Molecular Shape and Packing:
Molecular shape plays a significant role in how efficiently molecules pack together. Compact, symmetrical molecules tend to pack more closely, resulting in stronger intermolecular forces and higher melting points. Conversely, irregular shapes hinder efficient packing, leading to weaker interactions and lower melting points.
Example: Branched-chain alkanes generally have lower melting points than their straight-chain isomers because branching disrupts efficient packing.
3. Polarity and Intermolecular Force Strength:
The presence of polar bonds and the overall polarity of the molecule significantly influence the melting point. Polar molecules exhibit dipole-dipole forces in addition to LDFs, leading to higher melting points compared to nonpolar molecules of similar size. The strongest intermolecular force, hydrogen bonding, leads to exceptionally high melting points.
Example: Water (H₂O) has a relatively high melting point (0°C) due to extensive hydrogen bonding between its molecules. This contrasts with methane (CH₄), a nonpolar molecule of similar size, which has a much lower melting point (-182°C).
4. Network Covalent Solids:
A special case exists for network covalent solids like diamond and quartz (SiO₂). These substances have a three-dimensional network of strong covalent bonds extending throughout the entire crystal lattice. To melt these materials, all these strong covalent bonds must be broken, requiring an immense amount of energy, resulting in extremely high melting points.
Comparing Melting Points: Covalent vs. Ionic
While many covalent compounds have relatively low melting points, it's crucial to remember that this is not an absolute rule. The melting points of covalent compounds are considerably lower than those of most ionic compounds. This difference stems from the fundamental nature of the bonding:
-
Ionic compounds: Held together by strong electrostatic forces between oppositely charged ions. A substantial amount of energy is needed to overcome these forces, resulting in high melting points (often above 1000°C).
-
Covalent compounds: Held together by weaker intermolecular forces. Less energy is needed to overcome these forces, leading to a broader range of melting points, often significantly lower than ionic compounds.
Illustrative Examples: A Spectrum of Melting Points
Let's examine some examples to illustrate the diversity in melting points among covalent compounds:
| Compound | Melting Point (°C) | Intermolecular Forces | Notes |
|---|---|---|---|
| Methane (CH₄) | -182 | London Dispersion Forces | Simple, nonpolar molecule |
| Ethanol (C₂H₅OH) | -114 | Hydrogen Bonding, Dipole-Dipole | Hydrogen bonding significantly raises MP |
| Iodine (I₂) | 113 | London Dispersion Forces | Larger molecule, stronger LDFs |
| Diamond (C) | >3500 | Covalent Network | Extremely high due to strong network |
| Quartz (SiO₂) | 1713 | Covalent Network | Extremely high due to strong network |
Applications and Significance
Understanding the melting points of covalent compounds is crucial in numerous applications:
-
Material Science: Designing materials with specific melting points for various applications, like polymers with tailored properties.
-
Chemistry: Identifying and characterizing unknown substances based on their physical properties, including melting point.
-
Pharmaceutical Industry: Determining the melting point of drugs is vital for quality control and ensuring purity.
Conclusion: The Variable Melting Points of Covalent Compounds
In summary, the melting point of a covalent compound is not inherently high or low. It depends on a complex interplay of factors, including molecular size, shape, polarity, and the strength of the intermolecular forces present. While many covalent compounds exhibit low to moderate melting points due to weaker intermolecular interactions compared to ionic compounds, some, particularly network covalent solids, possess exceptionally high melting points due to strong covalent bonding throughout their structure. The diverse range of melting points observed in covalent compounds reflects the multifaceted nature of intermolecular forces and their influence on macroscopic properties. Understanding these factors is crucial for predicting and manipulating the properties of covalent materials across numerous fields.
Latest Posts
Latest Posts
-
Which Of The Following Is Not Correctly Matched
Mar 18, 2025
-
Balanced Equation Of H2so4 And Naoh
Mar 18, 2025
-
A Set That Contains No Elements Is Called The
Mar 18, 2025
-
How Many Electrons Are In One Coulomb
Mar 18, 2025
-
Amount Of Space Occupied By An Object
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is The Melting Point Of Covalent Compounds High Or Low . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
