How Many Electrons Are In One Coulomb
News Leon
Mar 18, 2025 · 5 min read
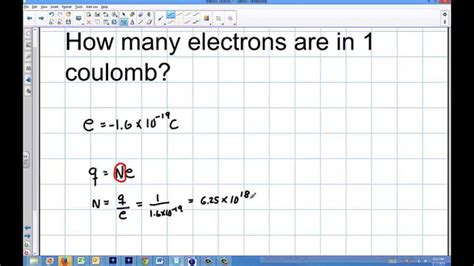
Table of Contents
How Many Electrons are in One Coulomb? A Deep Dive into Electrical Charge
The seemingly simple question, "How many electrons are in one coulomb?" opens a door to a fascinating exploration of fundamental physics, electricity, and the very nature of charge. While the answer itself is straightforward, understanding its implications requires delving into the concepts of charge quantization, Coulomb's Law, and the historical development of electrical units. This comprehensive guide will not only provide the answer but also explore the broader context of this crucial concept.
Understanding the Coulomb: A Unit of Electrical Charge
The coulomb (symbol: C) is the International System of Units (SI) unit of electric charge. It's a measure of the amount of electrical charge carried by a particular number of electrons (or protons). This is a crucial unit in electromagnetism, underpinning our understanding of electric current, electric fields, and many other electrical phenomena. Imagine it as a fundamental building block for quantifying electricity. One coulomb represents a significant amount of charge, and we'll soon see exactly how significant.
Charge Quantization: The Discrete Nature of Charge
A key concept to grasp is charge quantization. This principle states that electric charge exists in discrete units, meaning it's not continuous but comes in specific, indivisible packets. The smallest unit of charge is the elementary charge, carried by a single proton or electron. This elementary charge, denoted as e, has a magnitude of approximately 1.602 x 10⁻¹⁹ coulombs. This means that charge can only exist as multiples of this elementary charge – you can't have half an electron's worth of charge, for instance.
Calculating the Number of Electrons in One Coulomb
Now, armed with the elementary charge, we can calculate how many electrons constitute one coulomb. The calculation is straightforward:
Number of electrons = 1 Coulomb / Elementary charge
Substituting the values:
Number of electrons = 1 C / (1.602 x 10⁻¹⁹ C/electron)
Number of electrons ≈ 6.24 x 10¹⁸ electrons
Therefore, there are approximately 6.24 x 10¹⁸ electrons in one coulomb of negative charge. This is a staggeringly large number! To put this into perspective, this is roughly equivalent to the number of grains of sand on a beach, or even the number of stars in several galaxies.
The Significance of the Coulomb and Electron Count
This calculation isn't just a mathematical exercise. It has far-reaching implications across various scientific and engineering fields:
-
Understanding Electrical Current: Electric current, measured in amperes (A), is defined as the rate of flow of electric charge. One ampere is equal to one coulomb of charge passing a given point per second. Knowing the number of electrons in a coulomb helps us visualize the massive number of charged particles moving to create even a small current.
-
Electromagnetism and Coulomb's Law: Coulomb's Law describes the force between two charged objects. This force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. Understanding the magnitude of charge in coulombs is crucial for applying and interpreting Coulomb's Law in various scenarios, from atomic interactions to macroscopic electrical systems.
-
Capacitance and Charge Storage: Capacitors store electrical energy by accumulating charge on their plates. The capacitance of a capacitor is defined as the amount of charge stored per unit voltage. Understanding the relationship between coulombs and the number of electrons allows for precise calculations regarding energy storage and capacitor behavior.
-
Electronic Devices and Semiconductors: In the realm of electronics, the movement and manipulation of individual electrons are central to the functioning of transistors, integrated circuits, and countless other devices. The relationship between coulombs and electrons provides a macroscopic view of the microscopic charge transfers happening within these technologies.
Historical Context and Development of Electrical Units
The definition and standardization of the coulomb are the result of a long and fascinating history of scientific discovery. Early experiments with static electricity, culminating in the work of scientists like Charles-Augustin de Coulomb, laid the foundation for our understanding of electrical charge and forces. The choice of the coulomb as the unit of charge wasn't arbitrary; it was carefully chosen to align with other SI units and ensure consistency across physics and engineering disciplines.
Beyond the Simple Calculation: Further Considerations
While the calculation of 6.24 x 10¹⁸ electrons per coulomb is a good approximation, it’s important to note some nuances:
-
Accuracy of the Elementary Charge: The value of the elementary charge, e, is experimentally determined, and hence has a certain level of uncertainty. This uncertainty propagates into the calculation of the number of electrons per coulomb.
-
Positive Charge: While the calculation focuses on electrons, it's equally applicable to positive charges. One coulomb of positive charge would represent the same number of protons.
-
Context Matters: In some specific contexts, such as discussing individual atoms or molecules, dealing with the elementary charge e directly might be more appropriate than considering coulombs.
Practical Applications and Real-World Examples
Understanding the relationship between coulombs and electrons is not limited to theoretical physics. It has numerous practical applications:
-
Battery Capacity: Battery capacity is often expressed in milliampere-hours (mAh). This represents the total charge the battery can deliver. Knowing the electron count per coulomb helps understand the number of charge carriers involved in powering devices.
-
Lightning Strikes: A single lightning strike can involve the transfer of billions of coulombs of charge. Understanding this quantity allows for assessing the immense energy involved in such events and its potential impact.
-
Electroplating: In electroplating, the amount of metal deposited is directly related to the charge passed through the electrolyte. The relationship between coulombs and electrons is essential for controlling the thickness and quality of the deposited layer.
-
Medical Imaging: Techniques like MRI (Magnetic Resonance Imaging) rely on the interaction of magnetic fields with charged particles. Understanding the charge involved helps in optimizing image quality and resolving power.
Conclusion: A Fundamental Concept with Far-Reaching Implications
The seemingly simple answer to "How many electrons are in one coulomb?" – approximately 6.24 x 10¹⁸ – reveals a deep and rich understanding of fundamental physics, electrical phenomena, and the nature of charge itself. This quantity is not just a numerical value; it's a cornerstone for numerous applications across various scientific and engineering disciplines. From understanding the flow of electricity to designing sophisticated electronic devices and analyzing powerful natural phenomena, the significance of this seemingly small unit of charge cannot be overstated. This exploration underscores the importance of delving into the fundamentals, revealing the profound connections between seemingly disparate areas of science and technology.
Latest Posts
Latest Posts
-
Word That Has Two Different Meanings
Mar 18, 2025
-
Is Soil A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
What Does Isinstance Do In Python
Mar 18, 2025
-
What Is The Difference Between Political Parties And Interest Groups
Mar 18, 2025
-
Electromagnetic Radiation At Its Maximum Wavelength Is
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In One Coulomb . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
