Is Sugar Dissolved In Water A Chemical Change
News Leon
Mar 28, 2025 · 5 min read
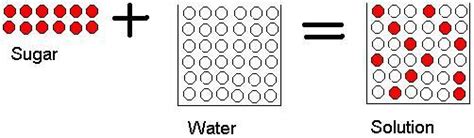
Table of Contents
Is Sugar Dissolved in Water a Chemical Change? A Deep Dive into Physical vs. Chemical Processes
The question of whether dissolving sugar in water constitutes a chemical change is a common point of confusion, even amongst those with a basic understanding of chemistry. While it might seem like a simple process, understanding the difference between physical and chemical changes is crucial for grasping the fundamental concepts of matter and its transformations. This article will delve deep into the intricacies of this seemingly simple phenomenon, exploring the defining characteristics of both physical and chemical changes and ultimately providing a definitive answer.
Understanding Physical and Chemical Changes
Before we tackle the sugar-water conundrum, let's establish a clear definition of physical and chemical changes. These two categories represent fundamentally different ways matter can be altered.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of it as reshaping the substance without fundamentally altering its identity. Examples include:
- Changes of state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid). The water molecule remains H₂O throughout these changes.
- Crushing or grinding: Breaking a rock into smaller pieces doesn't change the chemical makeup of the rock; it simply changes its size and shape.
- Dissolving (some cases): Many substances dissolve in water, creating a solution. However, it's crucial to note that not all dissolving processes are purely physical. We'll elaborate on this later.
- Mixing: Combining sand and water results in a mixture, but neither substance undergoes a chemical alteration.
The key takeaway here is that in physical changes, the substance's chemical formula and internal structure remain unchanged. You can often reverse a physical change by applying a different physical process (e.g., freezing liquid water to revert it to ice).
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms to form new substances with different chemical properties. This fundamentally alters the chemical composition of the matter involved. Chemical changes often involve:
- Formation of new substances: The products have different properties than the reactants.
- Energy changes: Chemical reactions often release (exothermic) or absorb (endothermic) energy in the form of heat, light, or electricity.
- Irreversibility (often): While some chemical changes can be reversed, many are irreversible without further chemical processes.
- Breaking and forming chemical bonds: This is the hallmark of a chemical change. Atoms are rearranged, leading to the creation of new molecules.
Examples include:
- Burning wood: Wood reacts with oxygen to produce ash, carbon dioxide, and water – entirely new substances with different chemical formulas.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a different chemical compound.
- Baking a cake: The ingredients undergo chemical reactions, resulting in a completely new substance with different properties.
Dissecting the Sugar-Water Solution
Now, let's return to our central question: is dissolving sugar in water a chemical change? The answer, in most cases, is no. Dissolving sugar in water is primarily a physical change.
Here's why:
- No new substance is formed: When sugar dissolves in water, the sugar molecules (sucrose, C₁₂H₂₂O₁₁) simply disperse amongst the water molecules (H₂O). The individual sugar and water molecules remain intact; they don't undergo a rearrangement of atoms to form new compounds. The chemical formula of the sugar and the water remains unchanged.
- The process is reversible: You can easily recover the sugar by evaporating the water. The sugar will be left behind, still having the same chemical composition as before. This reversibility is a hallmark of physical changes.
- No significant energy change: Dissolving sugar in water involves a small energy change (it might slightly cool or warm), but this is far less dramatic than the energy changes seen in chemical reactions like combustion or explosions. The energy involved is related to the breaking of intermolecular forces in the sugar crystal and the formation of new intermolecular forces between sugar and water molecules.
- Sugar molecules retain their identity: Spectroscopic analysis (techniques that analyze the interaction of electromagnetic radiation with matter) of the sugar solution would show the presence of unchanged sugar molecules. This indicates no new chemical species have been formed.
Exceptions and Nuances: When Dissolving Becomes Chemical
While dissolving sugar in water is generally a physical change, there are some nuanced situations where the line blurs:
-
Hydrolysis: Under specific conditions, especially with prolonged exposure to water and high temperatures, or in the presence of certain catalysts (such as acids), sucrose can undergo hydrolysis. Hydrolysis is a chemical reaction where water molecules break down a chemical compound. In the case of sucrose, hydrolysis breaks down sucrose into its constituent monosaccharides, glucose and fructose. This is a chemical change, as new chemical species (glucose and fructose) are formed. This is not a typical everyday scenario when dissolving table sugar, but it highlights the importance of considering the specific conditions.
-
Reactions with water: Some substances react chemically with water when dissolved. For example, certain metal oxides, like sodium oxide, react vigorously with water, forming a new substance (sodium hydroxide) and generating heat. This is a chemical change, not simply a dissolution process.
Practical Implications and Conclusion
Understanding the difference between physical and chemical changes is crucial in various fields, including:
- Cooking: Many cooking processes involve both physical and chemical changes. Knowing which is which helps in understanding the changes in the food's texture, taste, and nutritional value.
- Material science: Designing new materials requires a deep understanding of how substances interact and undergo transformations.
- Environmental science: Many environmental processes, like pollution and degradation, involve chemical changes that need to be understood to mitigate their effects.
In conclusion, dissolving table sugar in water is predominantly a physical change. The sugar molecules retain their chemical identity, no new substances are formed, the process is reversible, and the energy change is minimal. However, it's important to be aware of the exceptions – like hydrolysis under specific conditions – that can transform this seemingly simple process into a chemical change. Recognizing the nuances between physical and chemical processes is key to a deeper understanding of how matter behaves and interacts with its surroundings. This knowledge enhances our understanding across many scientific disciplines and everyday life applications. Therefore, careful consideration of the conditions and the substances involved is essential for accurate classification.
Latest Posts
Latest Posts
-
Both Glucose And Fructose Are
Mar 31, 2025
-
A Perfectly Elastic Demand Curve Implies That The Firm
Mar 31, 2025
-
Which Of The Following Are Complementary Bases In Dna
Mar 31, 2025
-
Classify The Following As A Homogeneous Or A Heterogeneous Mixture
Mar 31, 2025
-
What Physical Quantity Does The Slope Represent
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Sugar Dissolved In Water A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
