Is Sodium A Gas Liquid Or Solid
News Leon
Mar 27, 2025 · 5 min read
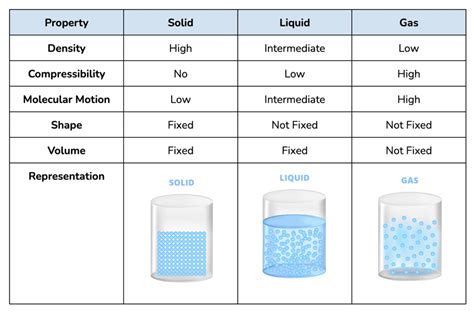
Table of Contents
Is Sodium a Gas, Liquid, or Solid? Understanding Sodium's Properties
Sodium (Na), a highly reactive alkali metal, is a fascinating element with unique properties that determine its state of matter. Understanding its physical characteristics is key to appreciating its role in various applications, from table salt to industrial processes. This comprehensive guide will delve deep into the physical characteristics of sodium, exploring its state at different temperatures and pressures, and its unique reactivity. We’ll also explore its atomic structure and how this influences its physical state.
Sodium's State at Room Temperature: A Solid Element
At standard room temperature (around 25°C or 77°F) and atmospheric pressure, sodium exists as a solid. This is a crucial point to remember. It's not a gas like helium or a liquid like mercury; it's a soft, silvery-white metal. Its solid nature is dictated by the strong metallic bonds holding its atoms together.
Understanding Metallic Bonding in Sodium
The atomic structure of sodium plays a pivotal role in determining its physical state. Sodium atoms have a single electron in their outermost shell. This electron is relatively loosely held and readily participates in metallic bonding. In a solid sodium sample, these outermost electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged sodium ions. This "sea" of electrons acts as a strong glue, binding the sodium ions together and creating a stable solid structure. This unique bonding mechanism is responsible for several of sodium's characteristic properties, including its excellent electrical and thermal conductivity.
The Crystal Structure of Solid Sodium
The sodium atoms within the solid arrange themselves in a highly organized manner, forming a body-centered cubic (BCC) crystal structure. This structure maximizes the efficient packing of sodium atoms while maintaining the strong metallic bonding. The regularity of this structure is reflected in the relatively high density of solid sodium compared to some other elements.
The Melting and Boiling Points of Sodium: Transitions Between States
While sodium is a solid at room temperature, its state can change if the temperature is altered significantly. Sodium's melting point is relatively low compared to many other metals. It melts at approximately 97.8°C (208°F). This relatively low melting point indicates that the metallic bonds, while strong enough to hold the atoms together in a solid at room temperature, are not exceptionally strong. This low melting point has implications for its handling and use in various industrial applications.
Above its melting point, sodium becomes a liquid. Liquid sodium, still silvery-white in appearance, retains its high thermal and electrical conductivity. This property makes liquid sodium an excellent heat transfer medium in certain industrial processes and nuclear reactors. It's important to note that liquid sodium is extremely reactive and requires specialized handling to prevent accidents.
Sodium's boiling point is much higher than its melting point, at approximately 883°C (1621°F). At this temperature, the kinetic energy of the sodium atoms overcomes the metallic bonds, and the liquid sodium transforms into a gas. Gaseous sodium is much less commonly encountered than its solid or liquid forms but is relevant in certain high-temperature industrial processes.
Sodium's Reactivity: A Key Factor Influencing its Handling
Sodium's high reactivity is a critical factor to consider when discussing its state. Its single valence electron is easily lost, leading to the formation of the Na+ ion. This makes sodium highly reactive with water, oxygen, and many other substances. This reactivity is why it's crucial to store sodium under inert conditions, usually in a mineral oil bath, to prevent unwanted reactions with air or moisture.
Sodium's Reaction with Water
One of the most dramatic demonstrations of sodium's reactivity is its reaction with water. When sodium is added to water, it reacts violently, producing hydrogen gas and sodium hydroxide (caustic soda). The reaction is highly exothermic, releasing significant heat and often igniting the hydrogen gas. This violent reaction underscores the need for extreme caution when handling sodium.
Sodium's Reaction with Oxygen
Sodium also readily reacts with oxygen in the air, forming sodium oxide (Na₂O). This oxide layer can further react with moisture and carbon dioxide in the air, forming a mixture of hydroxides and carbonates. This layer can protect the underlying sodium from further oxidation to a limited extent, but the overall reactivity means storage in inert conditions is necessary for long-term preservation.
Practical Applications of Sodium in Different States
The different states of sodium, and its unique properties in each state, determine its various applications:
Solid Sodium:
- Sodium lamps: Solid sodium is used in the manufacturing of high-pressure sodium-vapor lamps. These lamps produce a highly efficient and intense light, making them useful for street lighting and other applications requiring bright illumination. The solid sodium is heated and vaporized within the lamp to produce light.
- Sodium metal alloys: Sodium is alloyed with other metals to enhance certain material properties.
- Chemical reactant: Solid sodium is used as a reactant in several chemical processes for the synthesis of various organic compounds.
Liquid Sodium:
- Heat transfer medium: Liquid sodium is utilized as a coolant in some fast breeder nuclear reactors. Its excellent thermal conductivity and relatively low melting point make it suitable for this high-temperature application.
- Chemical reactant: Liquid sodium is employed in several industrial processes as a reducing agent.
Gaseous Sodium:
- Sodium vapor lamps: The gaseous state of sodium is directly involved in the light production within sodium vapor lamps as mentioned above. The high temperature within the lamp vaporizes the sodium, leading to light emission.
- Specialized chemical processes: Some high-temperature chemical processes utilize gaseous sodium as a reactant.
Conclusion: Understanding Sodium's multifaceted nature
Sodium, though seemingly simple, exhibits a fascinating range of properties and behaviors related to its state of matter. From its solid form at room temperature, with its unique metallic bonding and BCC crystal structure, to its liquid and gaseous states at higher temperatures, its applications span various industrial and technological fields. However, its significant reactivity necessitates careful handling and safe storage procedures. Understanding the transition between states, the underlying atomic structure, and its reactivity provides a complete picture of this essential and versatile element. This detailed analysis highlights the importance of considering an element's physical properties and chemical behavior when determining its applications and handling procedures. Further research into sodium's behavior under extreme conditions, such as high pressures, could reveal further insights into its complex nature.
Latest Posts
Latest Posts
-
Frame A Sentence Using The Word
Mar 30, 2025
-
The Broadest Taxonomic Group For Classifying Living Organisms Is The
Mar 30, 2025
-
Ca Oh 2 Hcl Balanced Equation
Mar 30, 2025
-
The Is The Fundamental Unit Of Life
Mar 30, 2025
-
The Lorenz Curve Represents The Relationship Between
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium A Gas Liquid Or Solid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
